Triplet therapy that inhibits the BRAF, MEK, and EGFR pathways appears promising in BRAF-mutated colorectal cancer, a malignancy that typically does not respond to BRAF inhibition alone.1 At the 2015 ASCO Annual Meeting, Chloe Evelyn Atreya, MD, of the University of California, San Francisco, presented results on 67 patients with BRAF-mutated colorectal cancer, a generally difficult patient subset to treat.
“These patients have a poor prognosis. With current chemotherapy regimens, median overall survival for BRAF-mutated colorectal cancer is only about 1 year, compared with 2.5 years in BRAF wild-type tumors,” she said.
Although BRAF is mutated in 50% of advanced melanomas, these mutations are observed in only about 8% of colorectal cancers. However, because colorectal cancer is common, there are about as many deaths associated with BRAF mutations in colorectal cancer as in melanoma, Dr. Atreya estimated.
Also, in contrast to BRAF-mutated melanomas, BRAF-mutated colorectal tumors are much less responsive to BRAF inhibition. Although about 80% of patients with BRAF-mutated melanoma respond to vemurafenib (Zelboraf), the response rate to the same drug is just 5% in colorectal cancer. “BRAF inhibition alone is not effective in BRAF-mutated colorectal cancer,” she noted.
Dr. Atreya and colleagues previously evaluated the benefit of dually targeting the MAP kinase pathway with both the BRAF inhibitor dabrafenib (Tafinlar) and the MEK inhibitor trametinib (Mekinist). This strategy only slightly improved outcomes, although one patient had a complete response that was ongoing for more than 3 years.
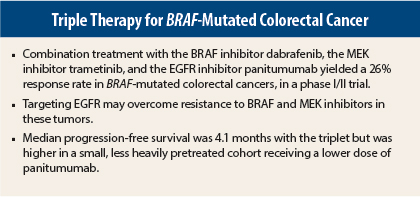
Biopsies indicated that dual targeting of the MAP kinase pathway failed to shut down phosphorylated ERK signaling completely. Therefore, their current study tests the strategy of also blocking upstream with the EGFR-targeted antibody panitumumab (Vectibix). Targeting the EGFR pathway may overcome resistance to BRAF and MEK inhibitors in these tumors, the investigators hypothesized.
Study Details
The phase I/II trial evaluated the combination of dabrafenib (150 mg twice daily) plus panitumumab (6 mg/kg every 2 weeks) in 20 patients with BRAF V600E-mutated metastatic colorectal cancer. The study also enrolled 35 patients on the triplet arm, which included dabrafenib, panitumumab, and trametinib in a dose-escalation scheme. Of these patients, 24 received the recommended phase II regimen of dabrafenib (150 mg twice daily) plus panitumumab (6 mg/kg every 2 weeks) and trametinib (2 mg daily).
The primary endpoint was overall response rate, and progression-free survival was a secondary endpoint.
“For completeness, we added two panitumumab-plus-trametinib cohorts, because secondary resistance to panitumumab or cetuximab is often mediated by alterations upstream of MEK in the MAP kinase pathway,” she said. She presented safety data on 14 of these patients.
Increased Response With Triplet
The evaluable population included 69 patients altogether who had received at least two prior treatment regimens. A total of 2 of 20 patients (10%) responded to the doublet of dabrafenib and panitumumab; 1 was a confirmed complete response. With triplet therapy, however, 9 of 35 patients (26%) responded, including 1 complete response. The unconfirmed response rate was 34%, and the stable disease rate was 60%, Dr. Atreya reported.
“Triplet therapy produced more durable disease control than dabrafenib plus panitumumab. Four triplet patients (11%) have been on study for more than 1 year,” she said.
Median progression-free survival was 3.4 months with the doublet and 4.1 months with the triplet. Median duration of response for all patients receiving the triplet was 5.4 months.
Median progression-free survival was considerably higher in the dose-escalation cohorts that included panitumumab at 4.8 mg/kg—approximately 9.5 months. “Although the numbers are very small, we note that these patients achieved higher panitumumab drug compliance and were exposed to fewer lines of prior therapy. The role of panitumumab dose and line of treatment, therefore, will be further investigated,” she added.
A pharmacokinetic analysis compared tumor biopsies before and after 2 weeks of treatment. Phosphorylated ERK staining intensity (a marker of inhibition of MAP kinase signaling) was consistently reduced by triplet therapy—but not doublet therapy—she reported, adding that the magnitude of change was less than that observed with dabrafenib alone in BRAF-mutated melanoma.
Adverse Events
The most common adverse events across all regimens were dermatitis acneiform and diarrhea. There were no drug-related grade 4-5 events.
Dermatologic toxicities were the most significant reason for dose interruptions, delays, and reductions. “Though the numbers are small, we have noted the greatest proportion of serious dermatologic toxicities with the combination of panitumumab and trametinib, without the BRAF inhibitor, suggesting the addition of dabrafenib may lessen these [side effects],” Dr. Atreya said.
To define the most effective and tolerable regimen, the researchers will expand the triplet cohort to evaluate two panitumumab doses and first vs later lines of therapy in 60 additional patients. They will also look further at the activity of panitumumab plus trametinib without dabrafenib. ■
Disclosure: Dr. Atreya has received research funding from GlaxoSmithKline and Novartis and has served as an advisor to Bayer Diagnostics.
Reference
1. Atreya CE, Van Cutsem E, Bendell JC, et al: Updated efficacy of the MEK inhibitor trametinib, BRAF inhibitor dabrafenib, and anti-EGFR antibody panitumumab in patients with BRAFV600E mutated metastatic colorectal cancer. 2015 ASCO Annual Meeting. Abstract 103. Presented May 31, 2015.