
Steven E. Vogl, MD
Persephone is a 4,088-patient trial that Helena Margaret Earl, MBBS, PhD, reported at the 2018 ASCO Annual Meeting as establishing that 6 months of trastuzumab (Herceptin) is not inferior to 12 months in 4-year survival without invasive or local regional recurrence or distant metastases. Dr. Earl called this outcome invasive disease–free survival. Part 1 of this column (page 63) describes the trial in detail and raises issues regarding its design, endpoint, and analysis. In part 2, I will explore these issues further in the context of 4 previously reported large trials that could not exclude inferiority for trastuzumab courses of less than 1 year.
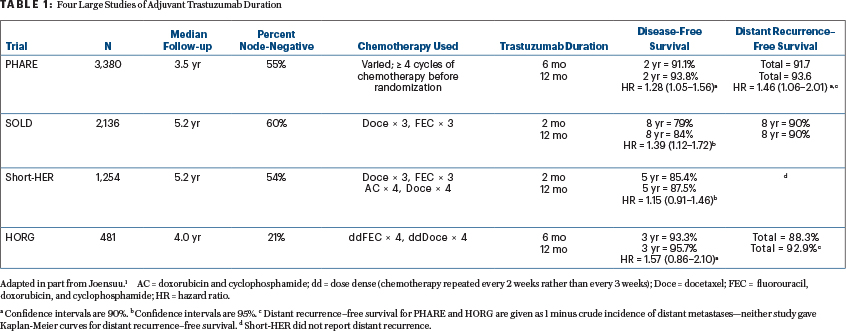
The 4 large randomized trials looking at trastuzumab duration with accruals over 300 subjects are listed in Table 1. The table excludes a 227-patient Eastern Cooperative Oncology Group (ECOG) trial in which longer-course trastuzumab produced more disease-free survival events than a shorter course and was associated with more deaths at a median follow-up of 6.4 years—presumably a fluke. This subject was recently reviewed1 in an article written before the results of two of the earlier trials had been published and before outcome results from Persephone had even been presented.
A recently published meta-analysis not including the just-published SOLD trial by Joensuu et al2 concluded that longer-duration trastuzumab therapy prolongs disease-free survival and overall survival.3 The trials in this meta-analysis (with 1 exception) have populations that are 40% to 60% node-negative, in the range of 59% for adjuvant therapy recipients in Persephone. Only Persephone included patients who were treated neoadjuvantly (15% of those entered).
Persephone is thus the outlier trial—it is the only one that concludes noninferiority. Then again, it is the biggest of the trials. Table 1 in part 1 of this column (page 64) outlines the features of -Persephone that could account for its outlier conclusion. I will here emphasize outcomes from PHARE and SOLD, not Short-HER and the Greek trial, for reasons specific to each trial.
The Greek trial, conducted by the Hellenic Oncology Research Group (HORG), had a major prognostic imbalance despite random assignment. Those assigned to longer therapy in that study had 50% more patients with negative nodes (25.3% vs 16.7%). While this difference failed to achieve statistical significance, it seems likely that it skewed the outcome. In addition, the HORG trial used docetaxel and FEC (fluorouracil, epirubicin, cyclophosphamide) every 14 days—a nonconventional schedule that most investigators find too toxic—and is relatively small.
The just-published Italian study of trastuzumab duration (Short-HER) was designed to use programs that had already been employed (a requirement of the Italian government, which sponsored the trial), so chemotherapy differed in the 9-week and 52-week arms, making any conclusions on trastuzumab duration suspect.4 For Short-HER, we have information on only disease-free survival and overall survival, not distant recurrence. Disease-free survival was improved by the regimen with longer trastuzumab therapy for those with stage III cancer (vs I and II, hazard ratio [HR] = 2.3, 90% confidence interval [CI] = 1.35–3.94) and those with nodal stages N2 and N3 (vs N0 and N1, HR = 2.25, 90% CI = 1.33–3.83). Disease-free survival was essentially identical for those with stage I cancers and those with either negative nodes or 1 to 3 positive nodes. A more detailed analysis presented at ESMO (October 19, 2018) reports that only women with a primary >2 cm and more than 4 involved nodes benefitted from longer trastuzumab. For the others, 5-year disease-free survival was 88% with 9 weeks of the drug and 89% with 52 weeks. Overall survival was essentially identical at 5 years (95.1 vs 95.0%), with superimposable survival curves out to 8 years.
Disease-Free Survival Is a Flawed Endpoint
After they concluded that they could not establish noninferiority for disease-free survival in their study of 3,380 French women, the PHARE investigators realized that disease-free survival is a flawed endpoint.5 Nine months later, they published a wonderful analysis in which they showed that simple anatomic staging could predict the benefit of 6 additional months of trastuzumab.6
The endpoint they chose for this subsequent analysis was metastasis-free survival, including metastases that developed after the occurrence of another disease-free survival event in a few cases. Metastasis, after all, is how breast cancer kills. Nonmetastatic events are often cured; metastatic ones are not. Their analysis is exploratory, not definitive, but the results suggest that reducing trastuzumab duration is dangerous for women with a higher risk of metastases.
Women With Small Node-Negative Cancers Did Great in PHARE
Women with tumors < 2.1 cm and negative nodes (33% of those entered) did great in PHARE regardless of the duration of trastuzumab treatment—98.3% metastasis-free survival at 3 years (Table 2). Women with either negative nodes and a primary > 2 cm or 1 to 3 positive nodes and a primary < 2.1 cm (37% of patients entered) had a small benefit (1.6%) from an extra 6 months of trastuzumab—95.8% vs 94.2% metastasis-free survival at 3 years (HR = 1.42). The 21% of women with 1 to 3 positive nodes and a primary > 2.0 cm or > 3 positive nodes and a primary < 2.1 cm had a greater benefit (4.7%)—metastasis-free survival at 3 years went from 85.7% to 90.4% with the extra 6 months of trastuzumab (HR = 1.37). Finally, the 10% of study subjects with primaries > 2.0 cm and > 3 positive nodes had the worst outcomes, but still benefited (3.6%) from prolonged trastuzumab—78.4% 3-year metastasis-free survival vs 74.8% (HR = 1.23).
The [PHARE] results suggest that reducing trastuzumab duration is dangerous for women with a higher risk of metastases.— Steven E. Vogl, MD
Tweet this quote
Because of the small numbers of events in PHARE, none of the differences within each prognostic group is statistically significant. The trend remains very interesting, though. A test for interaction suggests that the very low-risk group (node-negative and primary < 2.1 cm) has a different benefit from prolonged trastuzumab than the other groups. One suspects that PHARE results for low-risk patients seem worse than the 1% incidence of distant metastases at 7 years in the APT trial7 because the PHARE analysis included deaths from any cause in the endpoint. In APT, such deaths were twice as frequent as distant metastases.
I would predict that longer follow-up and the accumulation of more events will statistically confirm the conclusions the PHARE authors drew from their analysis: that there is a benefit to longer-duration trastuzumab but that it is clinically important only at higher levels of risk for metastasis. An 8-year analysis of PHARE has been completed and will be published shortly (Xavier Pivot, MD, PhD, personal communication, October 4, 2018). It is rumored to confirm the 3-year data.
Outcome for Small Node-Negative Cancers in Persephone Not Known
The presenter of Persephone, Dr. Earl, has noted (Helena Margaret Earl, personal communication, September 6, 2018) that a test for heterogeneity in Persephone failed to suggest a difference in disease-free survival according to nodal status (negative, 1–3 positive nodes, or > 3 positive nodes), or nodal status combined with estrogen receptor status (both positive, one positive, neither positive). The statisticians have not looked in detail at differences including primary tumor size as a variable, nor have they plotted disease-free survival curves for the subgroups. While such analyses would be exploratory, whether they confirm the PHARE observations is of great interest, especially with distant metastases as the endpoint.
The forest plots of invasive disease–free survival according to nodal status and treatment duration are promised for the upcoming publication. I hope Dr. Earl decides to publish the results for distant metastases and to include a detailed analysis by subgroups including primary tumor size, and especially the subgroup with negative nodes and small primary tumors, who did very well in France and in Boston.
SOLD Results Mixed
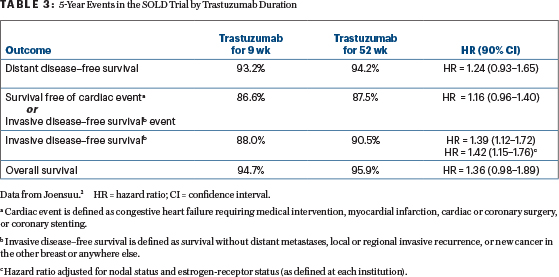
The results of the SOLD trial, a 2,174-patient Finnish study of 9 weeks vs 12 months of trastuzu-mab, suggest longer therapy improves disease-free survival but neither distant metastases nor overall survival (the latter Kaplan-Meier curves are almost superimposable). Although 12% of the trial population had advanced stage III cancers, 60% of those entered had negative nodes and 56% of primaries were < 2.1 cm.
In SOLD, centers that chose a docetaxel dose of only 80 mg/m2 had a significantly improved disease-free survival with 1 year of trastuzumab (rather than 9 weeks), compared to those that chose 100 mg/ m2, suggesting that the duration of trastuzumab and details of the chemotherapy interact in producing a favorable outcome. This may be restated as, “9 weeks of trastuzumab may be enough if given concurrently with aggressive chemotherapy.” This conclusion is based on a subgroup analysis and on treatment assignment that was not randomized and should be considered tentative at best.
The results of the SOLD trial … suggest longer [trastuzumab] therapy improves disease-free survival but neither distant metastases nor overall survival.— Steven E. Vogl, MD
Tweet this quote
The SOLD data suggest that 9 weeks of trastuzumab may be inferior to 1 year of trastuzumab in terms of invasive disease–free survival events (as defined in STEEP), but the 2 regimens seem pretty close in terms of survival free of distant recurrence, distant recurrence, and overall survival. Even though the latter 2 curves appear close together out to 9 years, the 90% confidence intervals just barely get below 1.00, suggesting that absolute superiority for a year of therapy is far from excluded, even for these endpoints (Table 3).
The SOLD results were not broken down to a low-risk group comparable to women entered in APT or those PHARE subjects with < 2.1-cm primaries and negative nodes. The SOLD investigators have promised an outcome analysis for this very low-risk group (Heikki Joensuu, personal communication, September 23, 2018). It will be of great interest if the gains for 52 weeks vs 9 weeks of trastuzumab prove trivial. However, for the primary invasive disease–free survival endpoint, longer therapy (1 year) seemed to be somewhat superior in a forest plot regardless of nodal status (negative, 1–3, or > 3 positive) or stage.
Change in Chemotherapy to Reduce Neuropathy Should Be Tested
Paclitaxel given for 12 weeks in the ECOG E1199 trial produced grade 2 or worse neuropathy in 20% of subjects and grade 3 or 4 neuropathy in 8%.8 Some of this is severe and long lasting.9,10 It would be worth testing a year of trastuzumab with chemotherapy that causes no long-term toxicity, like CMF (cyclophosphamide, methotrexate, fluorouracil). To my knowledge, no one is doing this, even though an analysis of the original Milan CMF trial11 showed CMF to be active in preventing the recurrence of cancers later found to be HER2-positive.
We Need Longer Follow-up
HER2-positive breast cancer is now a chronic disease among women in whom it is not cured. In the North Central Cancer -Treatment Group 9831 study, 10-year survival was 84% in a node-positive population. In CLEOPATRA, median survival in a protocol-eligible population after metastases were identified was 57 months.
It is naive to accept that shortening therapy is not deleterious when only 3- to 5-year follow-up is available. Even if concerns with Persephone are adequately answered in the final publication, four other trials have suggested that relapse rates are higher when the duration of trastuzumab therapy is truncated.
These women are being treated for cure, and we owe it to them, and to patients who will present in the future, to document their long-term outcomes. I hope the five larger trials we now have will be funded to continue to collect data on disease-free survival, distant recurrence, cardiac function, cardiac events, and death for at least another decade. The analyses should look at the interaction of outcome with trastuzumab duration, patient risk of distant recurrence on standard therapy, and chemotherapy program.

The goal should be to advise each patient of the likely absolute price to pay for shortening trastuzumab therapy, giving less toxic chemotherapy, or not taking an additional HER2-targeted agent. For each patient, the question should not be “is a short course of trastuzumab inferior?” but “how inferior is it for that patient?” This information will be difficult to acquire. The weight of the evidence so far is that 2 or 6 months of therapy is inferior to 12 months, but not very inferior at all for those with a very low risk in the first place.
Other studies have, and will, investigate small-molecule agents (like neratinib [Nerlynx] and lapatinib [Tykerb]) and monoclonal antibodies (like pertuzumab [Perjeta]) that may be used as partners or supplements to trastuzumab in this setting.
What Do We Do Now?
We look forward to a subset analysis of both disease-free survival and distant recurrence–free interval, based on primary tumor size and nodal status in the Persephone, PHARE, Short-HER, and SOLD trials, with longer follow-up.
Based on the PHARE subset analysis, I would be loath to give higher-risk women only 6 months of trastuzumab. A 3% to 5% higher rate of distant metastases at 3 years means at least 3% to 5% more deaths later on. A 1% to 2% increase in serious cardiac toxicity from longer trastuzumab therapy does not outweigh the benefit. The entire 10-year mortality reduction from the addition of trastuzumab to ACT (doxorubicin, cyclophosphamide, and paclitaxel) is 10% among women with involved axillary nodes.12 I would hate to lose half this benefit from a shorter trastuzumab course.
For the lower-risk women, I would still rather attenuate the chemotherapy (by deleting AC) than shorten the trastuzumab course. I would be reluctant to recommend doing both, or substituting CMF for paclitaxel, without a trial with adequate follow-up to show that giving the gentler chemotherapy with shorter trastuzumab does not reduce efficacy to an unacceptable level. We have suggestive evidence from SOLD that the duration of trastuzumab therapy interacts with the intensity of chemotherapy.
Having already achieved much in the treatment of what only 15 years ago was considered an especially aggressive and lethal form of breast cancer, we can and will achieve more.— Steven E. Vogl, MD
Tweet this quote
The addition of adjuvant pertuzumab is not clearly worthwhile based on early data from -APHINITY—the 4% reduction in 4-year distant metastases for the node-positive population is associated with a two-sided P value of only .10—the issue is still open.13,14 If pertuzumab works, how long to give it remains unknown. Also unknown is whether giving pertuzumab as an adjuvant treatment means we lose its dramatic effect on the prolongation of survival after metastases occur.
The very low-risk patients (defined by negative nodes and primaries < 2.1 cm) clearly need their own studies—the goal is to preserve the wonderful efficacy and reduce the toxicities most distressing to the patients. For the higher-risk women, the goals should be to enhance efficacy without a large price in immediate toxicity or in the quality of a life substantially prolonged by adjuvant therapy. Each higher-risk patient will have to weigh with her physician the benefits of more therapy (be it a second 6 months of trastuzumab, more aggressive or more toxic chemotherapy, or the addition of pertuzumab or neratinib) against the immediate- and long-term toxicity and, alas, the exorbitant costs that someone has to bear.
The current standard for women with involved axillary nodes, ACTH (doxorubicin, cyclophosphamide, and paclitaxel, with 1 year of trastuzumab), results in a 10-year overall survival of 84%! This is not a bad position from which to start discussion of the modification of therapy with a patient.
It seems likely that 2 to 6 months of trastuzu-mab is somewhat less effective than 12 months. We need to look at the extent of the absolute benefit of -longer therapy for each patient (which is dependent in part on the extent of the risk). Having already achieved much in the treatment of what only 15 years ago was considered an especially more aggressive and lethal form of breast cancer, we can and will achieve more. ■
DISCLOSURE: Dr. Vogl reported no conflicts of interest.
REFERENCES
1. Joensuu H: Escalating and de-escalating treatment in HER2-positive early breast cancer. Cancer Treat Rev 52:1-11, 2017.
2. Joensuu H, Fraser J, Wildiers H, et al: Effect of adjuvant trastuzumab for a duration of 9 weeks vs 1 year with concomitant chemotherapy for early human epidermal growth factor receptor 2-positive breast cancer: The SOLD randomized clinical trial. JAMA Oncol 4:1199-1206, 2018.
3. Gyawali R, Niraula S: Duration of adjuvant trastuzumab in HER2 positive breast cancer: Overall and disease free survival results from meta-analyses of randomized controlled trials. Cancer Treat Rev 60:18-23, 2017.
5. Pivot X, Romieu G, Debled M, et al: 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): A randomized phase 3 trial. Lancet Oncol 14:741-748, 2013.
6. Kramar A, Bachelot T, Madrange N, et al: Trastuzumab duration effects within patient prognostic subgroups in the PHARE trial. Ann Oncol 25:1563-1570, 2014.
7. Tolaney SM, Barry WT, Guo H, et al: Seven-year follow-up of adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. 2017 ASCO Annual Meeting. Abstract 511. Presented June 4, 2017.
8. Sparano JA, Wang M, Martino S, et al: Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 358:1663-1671, 2008.
9. Hershman DL, Weimer LH, Wang A, et al: Association between patient reported outcomes and quantitative sensory tests for measuring long-term neurotoxicity in breast cancer survivors treated with adjuvant paclitaxel chemotherapy. Breast Cancer Res Treat 125:767-774, 2011.
10. Hershman DL, Unger JM, Crew KD, et al: Two-year trends of taxane-induced neuropathy in women enrolled in a randomized trial of acetyl-L-carnitine (SWOG S0715). J Natl Cancer Inst 110:669-676, 2018.
11. Menard S, Valagussa P, Pilotti S, et al: Response to cyclophosphamide, methotrexate, and fluorouracil in lymph node-positive breast cancer according to HER2 overexpression and other tumor biologic variables. J Clin Oncol 19:329-335, 2001.
12. Perez EA, Romond EH, Suman VJ, et al: Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: Planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol 32:3744-3752, 2014.
13. von Minckwitz G, Procter M, de Azambuja E, et al: Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med 377:122-131, 2017.
14. Vogl SE: Adjuvant pertuzumab in HER2-positive breast cancer: Value yet to be demonstrated. The ASCO Post, October 10, 2017. Available at www.ascopost.com/issues/october-10-2017/adjuvant-pertuzumab-in-her2-positive-breast-cancer-value-yet-to-be-demonstrated. Accessed October 29, 2018.

