Renal cell carcinoma can be added to the growing list of tumors that respond to programmed death (PD)-1 immune checkpoint inhibitors, according to the results of the CheckMate trials, presented at the 2014 ASCO Annual Meeting.
The phase II CheckMate-010 trial evaluated three doses of nivolumab as a single agent in patients with previously treated renal cell carcinoma, while the phase Ib CheckMate-016 trial evaluated the drug at different doses and schedules in combination with other agents.
“The data from the trials are encouraging, as we seek to identify new treatments for patients, particularly those who show disease progression following treatment with antiangiogenic therapy, as these patients have limited options,” said Robert J. Motzer, MD, medical oncologist at Memorial Sloan Kettering Cancer Center, New York, who presented CheckMate-010.
Nivolumab as a Single Agent
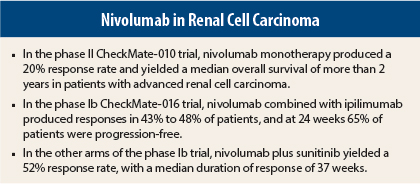
CheckMate-010 enrolled 168 patients with advanced disease who had received one to three prior systemic therapies, including at least one agent targeting the vascular endothelial growth factor (VEGF) pathway.1 Responses were observed in 20%, and median overall survival exceeded 2 years, Dr. Motzer reported (Table 1).
Treatment-related severe adverse events occurred in 7.2% of patients across all doses. The investigators observed no grade 3/4 pneumonitis.
Nivolumab Plus Ipilimumab
The phase Ib CheckMate-016 study evaluated the safety and tolerability of nivolumab at different doses and schedules in combination with other agents in both previously treated and treatment-naive patients. The data for nivolumab plus ipilimumab (Yervoy) in 44 patients was presented by Hans J. Hammers, MD, PhD, Assistant Professor of Oncology at the Sidney Kimmel Compehensive Cancer Center at Johns Hopkins Medicine, Baltimore.2
Patients were randomly assigned to one of two regimens, each given every 3 weeks for four cycles: nivolumab at 3 mg/kg plus ipilimumab at 1 mg/kg (arm N3+I1) or nivolumab at 1 mg/kg plus ipilimumab at 3 mg/kg (arm N1+I3). Both arms received maintenance nivolumab at 3 mg/kg every 2 weeks until disease progression. Patients were previously treated or treatment-naive.
“Nivolumab plus ipilimumab showed acceptable safety and evidence of antitumor activity in metastatic [renal cell carcinoma],” Dr. Hammers reported. “The objective response rate suggests greater activity than reported previously with either agent as monotherapy in [renal cell carcinoma].”
Confirmed responses were observed in 43% of the N3+I1 arm and in 48% of the N1+I3 arm. Median duration of response was 31.1 weeks with N3+I1 and was not reached in the other arm after 40 weeks of follow-up. Ongoing responses were observed in approximately 80% of the responders.
Median progression-free survival was 36.6 weeks and 38.3 weeks in the N3+I1 and N1+I3 arms, respectively, and at 24 weeks, approximately 65% of both arms were progression-free, he said.
“Grade 3/4 events were manageable within established treatment guidelines,” he said.
“The encouraging antitumor activity reported with this combination is the basis for a planned phase III combination trial in first-line metastatic renal cell carcinoma (CheckMate-214),” he said.
Other Nivolumab Combinations
Asim Amin, MD, PhD, medical oncologist at the Levine Cancer Institute, Carolinas HealthCare System, Charlotte, North Carolina, presented the findings for nivolumab in combination with a VEGF tyrosine kinase inhibitor, either sunitinib (Sutent) or pazopanib (Votrient). “Nivolumab plus a [tyrosine kinase inhibitor] showed encouraging activity, with a manageable toxicity profile. The objective responses rates were higher with the combinations than were previously seen with nivolumab or VEGF [tyrosine kinase inhibitor] monotherapy.”
The combination makes biologic sense, he commented. VEGF suppresses the tumor immune environment by inhibiting dendritic cell maturation and promoting myeloid-derived suppressor cells and regulatory T cells. VEGF tyrosine kinase inhibitors have been shown to reverse this immune suppression. Nivolumab blocks PD-1 from switching off T cells.
“In combination, we could give the VEGF [tyrosine kinase inhibitor] to condition the immune microenvironment and then give nivolumab for immune modulation,” he explained.
The phase Ib dose-escalating/expansion study evaluated nivolumab given either with sunitinib (n = 33) or pazopanib (n = 20). In the dose-escalation phase, patients were allowed prior treatment with one tyrosine kinase inhibitor. Based on the toxicities observed in the dose-escalation phase, the sunitinib arm proceeded to the expansion phase, which included treatment-naive patients.
The objective response rate was 52% with sunitinib and 45% with pazopanib; median duration of response was 37 weeks and 30 weeks, respectively, and ongoing responses were observed in 59% of responders to sunitinib/nivolumab (which included treatment-naive patients) and 33% of responders to pazopanib/nivolumab (the entire cohort had prior treatment). About one-third of each arm achieved stable disease.
“We saw robust responses across all three groups, without obvious differences related to prior treatment status,” Dr. Amin reported.
Median progression-free survival was 48.9 weeks with sunitinib and 31.4 weeks with pazopanib. Ongoing responses were observed in 58.8% and 33.3%, respectively.
Adverse events, however, were common, including grade 3/4 events in 82% receiving sunitinib and 70% receiving pazopanib, including 20% of patients with grade 3/4 elevation in transaminases. “This is more than we have previously seen with [tyrosine kinase inhibitors],” he noted. “Renal and hepatic adverse event [rates] were higher than expected with either agent alone.”
With sunitinib, increases in blood creatinine, autoimmune nephritis, and acute renal failure were observed in one patient each. More than one-third of the sunitinib arm discontinued therapy due to adverse events.
“The combination of a PD-1 inhibitor and a VEGF inhibitor warrants further investigation, but should be approached with caution,” Dr. Amin concluded. ■
Disclosure: Dr. Motzer is a consultant or advisor for AVEO, Bayer, Genentech, Merck, and Pfizer, has received research funding from Bristol-Myers Squibb, GlaxoSmithKline, Novartis, and Pfizer, and has given expert testimony for Pfizer. Dr. Hammers has received research funding from Bristol-Myers Squibb. Dr. Amin is a consultant or advisor for and has received honoraria and research funding from Bristol-Myers Squibb. For full disclosures of all study authors, visit meetinglibrary.asco.org.
References
1. Motzer RJ, Rini BI, McDermott DF, et al: Nivolumab for metastatic renal cell carcinoma: Results of a randomized, dose-ranging phase II trial. ASCO Annual Meeting. Abstract 5009. Presented May 31, 2014.
2. Hammers HJ, Plimack ER, Infante JR, et al: Phase I study of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma. ASCO Annual Meeting. Abstract 4504. Presented June 2, 2014.
3. Amin A, Plimack ER, Infante JR, et al: Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with sunitinib or pazopanib in patients with metastatic renal cell carcinoma. ASCO Annual Meeting. Abstract 5010. Presented May 31, 2014.