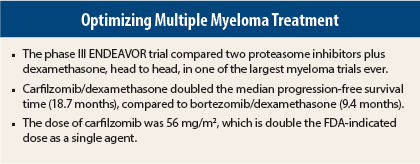
In the first head-to-head study comparing two proteasome inhibitors in relapsed multiple myeloma, carfilzomib (Kyprolis)/dexamethasone provided a doubling in progression-free survival, compared with bortezomib (Velcade)/dexamethasone.1 Results of the phase III ENDEAVOR trial of the two regimens in previously treated patients were reported at the 2015 ASCO Annual Meeting by Meletios A. Dimopoulos, MD, of the National and Kapodistrian University of Athens in Greece.
“In what is one of the largest trials ever done in multiple myeloma, carfilzomib/dexamethasone resulted in a twofold decrease in the risk of progression or death, compared with bortezomib/dexamethasone,” Dr. Dimopoulos said.
Median progression-free survival was 18.7 months with carfilzomib/dexamethasone vs 9.4 months with bortezomib/dexamethasone, for a hazard ratio (HR) of 0.53 (P < .0001).
Study Details and Results
ENDEAVOR included 929 multiple myeloma patients who had received one to three prior treatments. Treatment involved a higher dose of carfilzomib (56 mg/m2 on a 28-day cycle) than the U.S. Food and Drug Administration–approved dose for the drug as a single agent (27 mg/m2). This was based on observations that, in combination with dexamethasone, the 56-mg dose produced higher response rates, Dr. Dimopoulos pointed out. The bortezomib dose was 1.3 mg/m2 by intravenous bolus or subcutaneous injection, and the dexamethasone dose was 20 mg in each arm.
Approximately half the patients in each arm had received prior bortezomib and prior thalidomide (Thalomid); 38% had been treated with lenalidomide (Revlimid), and fewer than 1% had received carfilzomib.
At a median follow-up of 11.2 months, risk of progression was reduced by 47% in the carfilzomib/dexamethasone arm (P < .0001). All subgroups benefited more from the carfilzomib/dexamethasone regimen, with the exception of patients with baseline creatinine clearance < 30 mL/min, for whom the results were comparable between the arms.
“We see an early separation of the [progression-free survival] curve that improves over time, indicating the continuous favorable effect of [carfilzomib/dexamethasone] in all subgroups,” he commented.
Objective response rates were also significantly improved with carfilzomib/dexamethasone: 77%, vs 63% (P < .0001). Complete responses or better occurred in 13% vs 6% (P < .0001), and very good partial responses or better were seen in 54% and 29%, respectively (P < .0001). Median duration of response was 21.3 months with carfilzomib/dexamethasone and 10.4 months with bortezomib/dexamethasone.
Overall survival, a secondary endpoint, is not yet mature. Median overall survival at this point is 24.3 months with bortezomib/dexamethasone but has not been reached with carfilzomib/dexamethasone (HR = 0.79; P = .066).
Toxicity Profile
Adverse events grade ≥ 3 were seen in 73% with carfilzomib/dexamethasone and 67% with bortezomib/dexamethasone. Serious adverse events were seen in 48% and 36%, respectively, and four and three patients in each group died due to toxicity. Discontinuations related to toxicity were observed in about 15% per arm.
The carfilzomib/dexamethasone arm demonstrated higher rates of grade ≥ 3 hypertension (9% vs 3%), dyspnea (5% vs 2.2%), and cardiac failure (5% vs 1.8%) but significantly lower rates of peripheral neuropathy. Patients on carfilzomib/dexamethasone also had more pyrexia and cough.
“Patients stayed on [carfilzomib/dexamethasone] longer, for a median of 40 weeks vs 27 weeks, yet the adverse event rates were the same between the groups,” he noted.
Delving further into the occurrence of neuropathy, Dr. Dimopoulos reported grade ≥ 3 neuropathy in 5% of the bortezomib/dexamethasone arm compared to 1.3% of the carfilzomib/dexamethasone arm. Of particular interest, grade ≥ 2 neuropathy occurred in 32% and 6%, respectively (odds ratio [OR] = 0.14; P < .0001), “despite the fact that 79% of the bortezomib/dexamethasone group received subcutaneous bortezomib throughout their treatment,” he noted.
Other Key Data
The advantage of carfilzomib/dexamethasone was observed across all age groups, with hazard ratios of 0.58 for patients under age 65 and 0.53 for patients 65 to 74 years old. Interestingly, patients ≥ age 75 maintained a long progression-free survival, 18.7 months vs 8.9 months with bortezomib/dexamethasone, a 62% risk reduction with carfilzomib/dexamethasone. Adverse events were also consistent across the age groups, he added.
“The effect of prior exposure to bortezomib was a key question in the study,” he continued, “but this had no effect on our results.” Hazard ratios were 0.48 for the group naive to bortezomib and 0.56 for the group with prior bortezomib treatment.
“Carfilzomib/dexamethasone was superior to bortezomib/dexamethasone, regardless of age or prior bortezomib exposure, and represents a new standard of care,” Dr. Dimopoulos concluded. ■
Disclosure: Dr. Dimopoulos reported receiving fees for consulting or advising, and honoraria, from Celgene and Onyx.
Reference
1. Dimopoulos MA, Moreau P, Palumbo A, et al: Carfilzomib and dexamethasone vs bortezomib and dexamethasone in patients with relapsed multiple myeloma: Results from the phase III study ENDEAVOR. 2015 ASCO Annual Meeting. Abstract 8509. Presented June 2, 2015.