In the third prespecified interim analysis of the phase III KEYNOTE-564 trial,1 adjuvant treatment with the PD-1 inhibitor pembrolizumab significantly improved overall survival compared with placebo in patients with intermediate- or high-risk clear cell renal cell carcinoma (RCC). However, a second randomized trial, CheckMate 914: Part B,2 found that adjuvant treatment with the PD-1 inhibitor nivolumab failed to improve disease-free survival vs placebo in patients with localized RCC who were at high risk after nephrectomy. Both studies were presented at the 2024 ASCO Genitourinary Cancers Symposium.1,2
The results of KEYNOTE-564 were presented by lead study author Toni K. Choueiri, MD, FASCO, Director of the Lank Center for Genitourinary Oncology, Co-Leader of the Kidney Cancer Program, Senior Physician at Dana-Farber Cancer Institute, and Professor of Medicine at Harvard Medical School. At a median follow-up of 57.2 months, in the intention-to-treat (ITT) population, the median overall survival was not reached in either the pembrolizumab or placebo arms, but the risk of death was reduced by 38% with adjuvant pembrolizumab (P = .002). At 48 months, the estimated overall survival rate was 91.2% with pembrolizumab vs 86% with placebo.

Toni K. Choueiri, MD, FASCO
“This is the first study to show a statistically significant and clinically meaningful survival improvement with any adjuvant therapy in kidney cancer. This further supports adjuvant pembrolizumab as a standard of care after surgery in this disease setting,” said Dr. Choueiri.
“In patients with clear cell RCC at increased risk of recurrence following surgery, there was a 38% reduction in the risk of death with adjuvant pembrolizumab, with a survival benefit seen across key subgroups. A continued disease-free survival benefit was demonstrated,” he continued. “Considering that many patients with clear cell renal cell carcinoma have a high risk of recurrence leading to noncurable distant metastases, this finding is practice-changing. The survival curves separate early and continue to separate over the longer term.”
Dr. Choueiri reminded listeners that more than 17 randomized controlled trials of adjuvant therapy have been previously conducted. “None has shown an improvement in survival,” he noted, and that includes CheckMate 914 (see discussion of Part B to follow).2
KEYNOTE-564: Details and Outcomes
The randomized, double-blind, multicenter KEYNOTE-564 trial enrolled patients with histologically confirmed clear cell RCC who had undergone surgery up to 12 weeks earlier and had not received prior systemic therapy. Patients (n = 994) were randomly assigned 1:1 to receive pembrolizumab at 200 mg or placebo every 3 weeks for up to 17 cycles. Patients were classified as being at intermediate-high or high risk of recurrence. Intermediate-high risk was defined as pT2 or pT3; high-risk disease was defined as pT4 or any pT with positive nodes.
Stratification factors included M stage, Eastern Cooperative Oncology Group (ECOG) performance status (0 vs 1), and location (United States vs outside the United States). Secondary endpoints were overall survival and safety.
Baseline characteristics were generally well balanced between the two arms. The median patient age was 60. Almost three-quarters were male and came from outside the United States. About 94% had no evidence of metastasis, and about 85% had an ECOG performance status of 0. About 85% were at intermediate-high risk of recurrence; 5% had M1 with no evidence of disease after reception. Sarcomatoid features were present in about 11%; about 25% had a combined positive score (CPS) of PD-L1 expression > 1%.
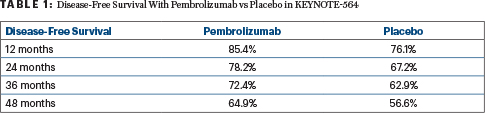
At the first prespecified analysis of the trial, adjuvant pembrolizumab achieved a significant benefit in disease-free survival vs placebo—a 32% improvement (hazard ratio = 0.68; 95% confidence interval = 0.53–0.87; P = .002).3 At the third interim analysis, median disease-free survival was not reached in either group. However, the rates favored adjuvant pembrolizumab, with a 28% risk reduction for pembrolizumab vs placebo (Table 1).
All subgroups experienced a disease-free survival benefit with adjuvant pembrolizumab vs placebo. The benefit was more pronounced in those with M1 no evidence of disease, M0 high-risk disease, and sarcomatoid features as well as those aged 65 or younger.
Recurrences were reported in 79.5% of the pembrolizumab-treated patients vs 81.4% of placebo patients. About 80% of patients went on to receive subsequent therapy, which included radiation, surgery, and/or systemic anticancer therapy.
“The question is after 57.2 months of follow-up: Is there any impact [of pembrolizumab] in terms of toxicity?” Dr. Choueiri asked. “With 27.1 [more months of] follow-up, there was no difference.”
Serious adverse events occurred in 20.7% of patients treated with pembrolizumab and 11.5% of those given placebo, leading to treatment discontinuation in 10.0% vs 1.0%, respectively. No deaths were attributed to treatment-related adverse events.
CheckMate 914: Part B
As previously mentioned, a second randomized trial presented at the 2024 ASCO Genitourinary Cancers Symposium found that adjuvant treatment with nivolumab failed to improve disease-free survival vs placebo in patients with localized RCC who were at high risk after nephrectomy,2 suggesting the positive results with pembrolizumab are not generalizable to nivolumab.
In Part A of CheckMate 914, nivolumab plus ipilimumab did not improve disease-free survival vs placebo. In Part B, which was designed to compare nivolumab monotherapy with placebo, at a median follow-up of 27 months, median disease-free survival was not reached in the nivolumab or placebo arm. At 18 months, disease-free survival rates were 78.4% with nivolumab and 75.4% with placebo.
“The primary endpoint of disease-free survival for nivolumab monotherapy was not met,” said lead author Robert J. Motzer, MD, of Memorial Sloan Kettering Cancer Center, New York.

Robert J. Motzer, MD
Part B included 835 patients; 411 were assigned to nivolumab monotherapy, 208 were assigned to placebo, and 206 were assigned to nivolumab plus ipilimumab. Median patient age was 59. Almost all patients (93%) underwent nephrectomy. About 80% had pT3 tumors. About 8% had sarcomatoid features, and about 10% had PD-L1 expression > 1%. A trend was observed toward nivolumab benefit in patients with sarcomatoid features, patients with PD-L1 expression > 1%, and those with a lower hemoglobin at baseline.
An important consideration about this study, Dr. Motzer told The ASCO Post, “is that the treatment course was 6 months and the trial was designed with 60% power, since it was originally designed to support CheckMate 214, which had a primary endpoint of assessing nivolumab plus ipilimumab vs placebo.”
DISCLOSURE: Dr. Choueiri owns stock in or has received honoraria from Curesponse, Osel, Pionyr, Precede Bio, and Tempest Therapeutics; has served as a consultant or advisor to Alkermes, Analysis Group, Aravive, Arcus Biosciences, ASCO, AstraZeneca, Bayer, Bristol Myers Squibb, Clinical Care Options, Eisai, EMD Serono, ESMO, Exelixis, Foundation Medicine, Gilead Sciences, GlaxoSmithKline, Harborside Press, Infinity Pharmaceuticals, Ipsen, Janssen Oncology, Kanaph Therapeutics, The Lancet Oncology, Eli Lilly, Merck, MJH Life Sciences, Navinata Health, NCCN, NiKang Therapeutics, Novartis, Peloton Therapeutics, Pfizer, PlatformQ Health, Precede Bio, Prometheus, Roche/Genentech, Sanofi/Aventis, Scholar Rock, Tempest Therapeutics, The New England Journal of Medicine, and UpToDate; has received research funding from Agensys, Arcus Biosciences, AstraZeneca, AVEO, Bayer, Bristol Myers Squibb, Calithera Biosciences, Eisai, Exelixis, GlaxoSmithKline, Ipsen, Merck, NiKang Therapeutics, Novartis, Peloton Therapeutics, Pfizer, Roche, Roche/Genentech, Seattle Genetics/Astellas, Takeda, and TRACON Pharma; holds patents and royalties from Biomarkers of Clinical Response and Benefit to Immune Checkpoint Inhibitor Therapy, ctDNA technologies, and PBRM1 Biomarkers Predictive of Anti-Immune Checkpoint Response; and has received travel expenses from Alexion Pharmaceuticals, Allegiant, Analysis Group, AstraZeneca, Bayer, Bristol Myers Squibb, Cerulean Pharma, Clinical Care Options, Corvus Pharmaceuticals, Eisai, EMD Serono, ESMO, Exelixis, Foundation Medicine, GlaxoSmithKline, Harborside Press, HERON, Ipsen, Kidney Cancer Association, The Lancet Oncology, Eli Lilly, Lpath, Merck, MJH Life Sciences, Navinata Health, NCCN, Novartis, Peloton Therapeutics, Pfizer, PlatformQ Health, Prometheus, Roche/Genentech, Sanofi/Aventis, The New England Journal of Medicine, and UpToDate. Dr. Motzer has received clinical research support from AVEO Pharmaceuticals, Bristol Myers Squibb, Eisai, Exelixis, Merck & Co, and Pfizer; and has served as a scientific advisor or consultant to Merck & Co and Takeda Pharmaceuticals North America.
REFERENCES
1. Choueiri TK, Tomczak P, Park SH, et al: Overall survival results from the phase 3 KEYNOTE-564 study of adjuvant pembrolizumab versus placebo for the treatment of clear cell renal cell carcinoma. 2024 ASCO Genitourinary Cancers Symposium. Abstract LBA359. Presented January 27, 2024.
2. Motzer RJ, Bex A, Russo P, et al: Adjuvant nivolumab monotherapy vs placebo for localized renal cell carcinoma at high risk of relapse after nephrectomy: Results from Part B of the randomized, phase 3 CheckMate 914 trial. 2024 ASCO Genitourinary Cancers Symposium. Abstract LBA358. Presented January 27, 2024.
3. Choueiri TK, Tomczak P, Park SH, et al: Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med 385:683-694, 2021.

