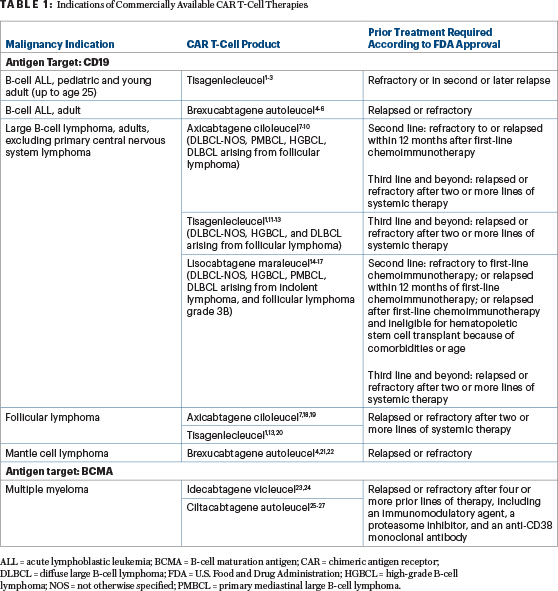
The ASCO Post is pleased to present Hematology Expert Review, an ongoing feature that quizzes readers on issues in hematology. With this installment, we launch a new series of articles on commercially available chimeric antigen receptor (CAR) T-cell therapies. Syed Ali Abutalib, MD, and Jennifer N. Brudno, MD, discuss how CAR T-cell therapy has become a major player in certain hematologic cancers, in part, by highlighting its indications, efficacy, and toxicities. This first installment addresses the indications for (Table 1)1-29 and trends in usage of (Figure 1)30 commercially available CAR T-cell therapies.
Question 1
Which of the following statements about 2021 data from the Cellular Immunotherapy Data Resource (CIDR), an initiative of the Center for International Blood and Marrow Transplant Research (CIBMTR), is correct?
A. Data collection on CAR T cells is voluntary, and capture of the overall activity of these therapies in the United States by the CIBMTR is estimated to encompass 95% of commercial products.
B. The most common indication for CAR T cells is acute lymphoblastic leukemia.
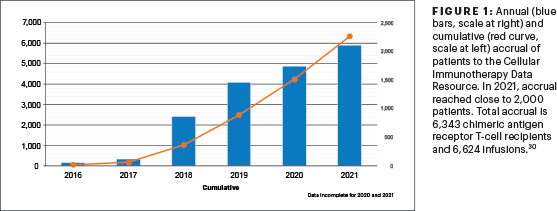
C. Annual and cumulative accrual of patients to the CIDR in 2021 reached close to 2,000 patients.
D. There has been a decline in the use of CAR T cells for mantle cell lymphoma and multiple myeloma.
Question 2
Which of the following commercially available CAR T-cell therapies is approved by the U.S. Food and Drug Administration (FDA) as a second-line therapy for delayed (> 12 months) relapse of large B-cell lymphoma in patients who are ineligible for autologous hematopoietic cell transplant?
A. Tisagenlecleucel
B. Axicabtagene ciloleucel
C. Lisocabtagene maraleucel
D. B and C
E. None of the above
Question 3
Which of the following commercially available CAR T-cell therapies is/are approved as second-line therapy for large B-cell lymphoma?
A. Tisagenlecleucel
B. Axicabtagene ciloleucel
C. Lisocabtagene maraleucel
D. B and C
E. None of the above
Question 4
Which of the following commercially available CAR T-cell therapies is not approved for relapsed primary mediastinal large B-cell lymphoma?
A. Tisagenlecleucel
B. Axicabtagene ciloleucel
C. Lisocabtagene maraleucel
D. None of the above
Question 5
Which of the following commercially available CAR T-cell therapies has/have an indication in relapsed or refractory grade 3A follicular lymphoma?
A. Tisagenlecleucel
B. Axicabtagene ciloleucel
C. Lisocabtagene maraleucel
D. Brexucabtagene autoleucel
E. A and B
F. A, B, and C
Question 6
Which of the following commercially available CAR T-cell therapies has an indication for relapsed or refractory B-cell precursor acute lymphoblastic leukemia in a 30-year-old patient.
A. Tisagenlecleucel
B. Axicabtagene ciloleucel
C. Lisocabtagene maraleucel
D. Brexucabtagene autoleucel
Question 7
CAR T-cell therapy is FDA-approved for which of the following indications?
A. Burkitt lymphoma
B. Primary central nervous system
lymphoma
C. Marginal zone lymphoma
D. Chronic lymphocytic leukemia
E. Multiple myeloma
Answers to Hematology Expert Review Questions
GUEST EDITORS

Syed Ali Abutalib, MD

Jennifer N. Brudno, MD
Question 1
Which of the following statements about 2021 data from the Cellular Immunotherapy Data Resource (CIDR), an initiative of the Center for International Blood and Marrow Transplant Research (CIBMTR), is correct?
Correct answer: C. Annual and cumulative accrual of patients to the CIDR in 2021 reached close to 2,000 patients.
Expert Perspective
The CIBMTR launched the CIDR cellular therapy registry in 2016 with the objective of capturing the demographics, patterns of use, and outcomes of recipients of cellular therapies. Similar to the hematopoietic cell transplant outcomes database, the cellular therapy registry captures long-term outcomes of recipients of these therapies, and it can be used to meet regulatory requirements for 15 years of follow-up, applicable to recipients of genetic-modified cellular therapies.
Data collection for CAR T cells is voluntary, and the capture of the overall activity of these therapies in the United States by the CIBMTR is estimated to be 65% of commercial products. This estimate is based on reconciling the number of products delivered to centers and subsequent reporting to the CIBMTR, using product identifiers.
Annual and cumulative accrual to the CIDR of patients in 2021 reached close to 2,000 patients (Figure 1). The most common indication for CAR T cells is large B-cell lymphoma, followed by acute lymphoblastic leukemia and multiple myeloma. There is a rapid expansion of CAR T cells being used for mantle cell lymphoma and multiple myeloma, aligning with the recent approvals.
Question 2
Which of the following commercially available CAR T-cell therapies is approved by the U.S. Food and Drug Administration (FDA) as a second-line therapy for delayed (> 12 months) relapse of large B-cell lymphoma in patients who are ineligible for autologous hematopoietic cell transplant?
Correct answer: C. Lisocabtagene maraleucel
Expert Perspective
Unlike tisagenlecleucel and axicabtagene ciloleucel, lisocabtagene maraleucel is also approved for delayed (> 12 months) relapse of large B-cell lymphoma after first-line chemoimmunotherapy in patients who are not eligible for hematopoietic stem cell transplantation because of comorbidities or age.
Question 3
Which of the following commercially available CAR T-cell therapies is/are approved as second-line therapy for large B-cell lymphoma?
Correct answer: D. B and C [axicabtagene ciloleucel and lisocabtagene maraleucel]
Expert Perspective
Axicabtagene ciloleucel and lisocabtagene maraleucel are both approved for second-line treatment of large B-cell lymphoma based on the ZUMA-7 and TRANSFORM trials,8,16,17 respectively. Both products were found to have an event-free survival advantage over standard-of-care platinum-based chemoimmunotherapy followed by high-dose chemotherapy and autologous stem cell transplant in responders. Axicabtagene ciloleucel additionally was found to have an overall survival benefit in ZUMA-7.28 The BELINDA trial29 did not show an event-free survival advantage of tisagenlecleucel compared with salvage chemotherapy followed by autologous stem cell transplant.
Question 4
Which of the following commercially available CAR T-cell therapies is not approved for relapsed primary mediastinal large B-cell lymphoma?
Correct answer: A. Tisagenlecleucel
Expert Perspective
Tisagenlecleucel is not approved for relapsed primary mediastinal large B-cell lymphoma. The JULIET trial,11,12 a phase II multicenter study evaluating tisagenlecleucel for patients with large B-cell lymphoma after two prior lines of therapy, excluded patients with primary mediastinal large B-cell lymphoma.
Question 5
Which of the following commercially available CAR T-cell therapies has/have an indication in relapsed or refractory grade 3A follicular lymphoma?
Correct Answer: E. A and B [tisagenlecleucel and axicabtagene ciloleucel]
Expert Perspective
Tisagenlecleucel and axicabtagene ciloleucel are approved for the treatment of relapsed or refractory follicular lymphoma (grades 1 to 3A) in adults after at least two lines of systemic therapy. Neither lisocabtagene maraleucel nor brexucabtagene autoleucel is approved for follicular lymphoma of any grade.
Lisocabtagene maraleucel is approved for the treatment of relapsed or refractory large B-cell lymphoma, including follicular lymphoma grade 3B in adults who have disease that is refractory to first-line chemoimmunotherapy or has relapsed within 12 months of first-line chemoimmunotherapy; disease that is refractory to first-line chemoimmunotherapy or has relapsed after first-line chemoimmunotherapy and who are not eligible for hematopoietic stem cell transplantation because of comorbidities or age; or relapsed or refractory disease after two or more lines of systemic therapy. Brexucabtagene autoleucel is approved for treatment of relapsed or refractory mantle cell lymphoma.
Question 6
Which of the following commercially available CAR T-cell therapies has an indication for relapsed or refractory B-cell precursor acute lymphoblastic leukemia in a 30-year-old patient.
Correct answer: D. Brexucabtagene autoleucel
Expert Perspective
Brexucabtagene autoleucel is approved for treatment of relapsed or refractory B-cell precursor acute lymphoblastic leukemia in adults. Tisagenlecleucel is also approved for the treatment of B-cell precursor acute lymphoblastic leukemia that is refractory or in second or later relapse—but only in patients up to age 25.
Question 7
CAR T-cell therapy is FDA-approved for which of the following indications?
Correct answer: E. Multiple myeloma
Expert Perspective
At the time of this writing, relapsed or refractory multiple myeloma is the only disease of the choices provided for which two FDA-approved CAR T-cell therapy products are available. The indications of commercially available CAR T-cell therapies are shown in Table 1.
DISCLOSURE: Dr. Abutalib has served on the advisory board for AstraZeneca. Dr. Brudno has served on the advisory board for Kyverna Therapeutics.
REFERENCES
1. Kymriah (tisagenlecleucel) [package insert]. Novartis Pharmaceuticals. Available at https://www.fda.gov/media/107296/download. Accessed July 5, 2023.
2. Maude SL, Laetsch TW, Buechner J, et al: Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 378:439-448, 2018.
3. Laetsch TW, Maude SL, Rives S, et al: Three-year update of tisagenlecleucel in pediatric and young adult patients with relapsed/refractory acute lymphoblastic leukemia in the ELIANA trial. J Clin Oncol 41:1664-1669, 2023.
4. Tecartus (brexucabtagene autoleucel) [package insert]. Kite Pharma, a Gilead company. Available at https://www.fda.gov/media/140409/download. Accessed July 5, 2023.
5. Shah BD, Ghobadi A, Oluwole OO, et al: KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: Phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet 398:491-502, 2021.
6. Shah BD, Ghobadi A, Oluwole OO, et al: Two-year follow-up of KTE-X19 in patients with relapsed or refractory adult B-cell acute lymphoblastic leukemia in ZUMA-3 and its contextualization with SCHOLAR-3, an external historical control study. J Hematol Oncol 15:170, 2022.
7. Yescarta (axicabtagene ciloleucel) [package insert]. Kite Pharma, a Gilead company. Available at https://www.fda.gov/media/108377/download. Accessed July 5, 2023.
8. Locke FL, Miklos DB, Jacobson CA, et al: Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med 386:640-654, 2022.
9. Neelapu SS, Locke FL, Bartlett NL, et al: Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 377:2531-2544, 2017.
10. Neelapu SS, Jacobson CA, Ghobadi A, et al: Five-year follow-up of ZUMA-1 supports the curative potential of axicabtagene ciloleucel in refractory large B-cell lymphoma. Blood 141:2307-2315, 2023.
11. Schuster SJ, Bishop MR, Tam CS, et al: Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 380:45-56, 2019.
12. Schuster SJ, Tam CS, Borchmann P, et al: Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 22:1403-1415, 2021.
13. Chong EA, Ruella M, Schuster SJ, et al: Five-year outcomes for refractory B-cell lymphomas with CAR T-cell therapy. N Engl J Med 384:673-674, 2021.
14. Breyanzi (brexucabtagene autoleucel) [package insert]. Bristol Myers Squibb. Available at https://www.fda.gov/media/145711/download. Accessed May 4, 2023.
15. Kamdar M, Solomon SR, Arnason J, et al: Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM). Lancet 399:2294-2308, 2022.
16. Abramson JS, Solomon SR, Arnason J, et al: Lisocabtagene maraleucel as second-line therapy for large B-cell lymphoma: Primary analysis of the phase 3 TRANSFORM study. Blood 141:1675-1684, 2023.
17. Abramson JS, Palomba ML, Gordon LI, et al: Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001). Lancet 396:839-852, 2020.
18. Jacobson CA, Chavez JC, Sehgal AR, et al: Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5). Lancet Oncol 23:91-103, 2022.
19. Palomba ML, Ghione P, Patel AR, et al: A 24-month updated analysis of the comparative effectiveness of ZUMA-5 (axi-cel) vs SCHOLAR-5 external control in relapsed/refractory follicular lymphoma. Expert Rev Anticancer Ther 23:199-206, 2023.
20. Fowler NH, Dickinson M, Dreyling M, et al: Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: The phase 2 ELARA trial. Nat Med 28:325-332, 2022.
21. Wang M, Munoz J, Goy A, et al: KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med 382:1331-1342, 2020.
22. Wang M, Munoz J, Goy A, et al: Three-year follow-up of KTE-X19 in patients with relapsed/refractory mantle cell lymphoma, including high-risk subgroups, in the ZUMA-2 study. J Clin Oncol 41:555-567, 2023.
23. Abecma (idecabtagene vicleucel) [package insert]. Bristol Myers Squibb. Available at https://www.fda.gov/media/147055/download. Accessed May 4, 2023.
24. Munshi NC, Anderson LD Jr, Shah N, et al: Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med 384:705-716, 2021.
25. Carvykti (ciltacabtagene autoleucel) [package insert]. Janssen Biotech, Inc. Available at https://www.fda.gov/media/156560/download. Accessed July 5, 2023.
26. Berdeja JG, Madduri D, Usmani SZ, et al: Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet 398:314-324, 2021.
27. Martin T, Usmani SZ, Berdeja JG, et al: Ciltacabtagene autoleucel, an anti-B-cell maturation antigen chimeric antigen receptor T-cell therapy, for relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J Clin Oncol 41:1265-1274, 2023.
28. Westin JR, Oluwole OO, Kersten MJ, et al: Survival with axicabtagene ciloleucel in large B-cell lymphoma. N Engl J Med 389:148-157, 2023.
29. Bishop MR, Dickinson M, Purtill D, et al: Second-line tisagenlecleucel or standard care in aggressive B-cell lymphoma. N Engl J Med 386:629-639, 2022.
30. Moskop, A, Jacobs B, Pasquini MC: Current uses of CAR T-cell therapies in the US: CIDR summary slides, 2021. Available at http://www.cibmtr.org. Accessed July 5, 2023.

