In the treatment of patients with advanced melanoma containing BRAF mutations, initial treatment with an immunotherapy combination achieved superior overall survival compared with targeted therapy in the phase III DREAMseq trial, also known as the ECOG-ACRIN EA6134 trial. The results were reported during the ASCO Plenary Series by Michael B. Atkins, MD, Deputy Director of the Georgetown Lombardi Comprehensive Cancer Center and William M. Scholl Professor of Oncology at Georgetown University Medical Center, Washington, DC.1
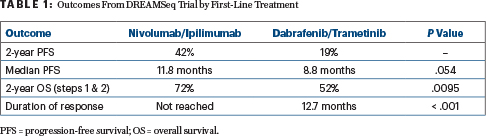
The sequence involving initial treatment with nivolumab plus ipilimumab resulted in an absolute 20% improvement in 2-year overall survival (P = .0095) over initial treatment with the BRAF/MEK inhibitor regimen of dabrafenib plus trametinib. The results triggered an early halt to the study after the fourth interim analysis, when just 59% of proposed accrued patients had reached the 2-year endpoint.
“In this population with oncogene-driven tumors and an effective targeted therapy, nivolumab/ipilimumab followed by BRAF/MEK inhibitors, if necessary, should be the preferred treatment sequence,” Dr. Atkins said.

Michael B. Atkins, MD

Lynn Schuchter, MD
ASCO spokesperson Lynn Schuchter, MD, Chief of Hematology/Oncology and Director of the Tara Miller Melanoma Center at the Abramson Cancer Center, University of Pennsylvania, Philadelphia, said the results of DREAMseq answer one of the most important clinical questions in melanoma care.
“For patients with melanoma and a BRAF V600 mutation who have not received prior therapy, the results clearly show better overall survival when combination immunotherapy is selected first. In an era of remarkable advances for patients with melanoma, this study is an important addition to understanding the best approach to provide the very best care,” Dr. Schuchter commented.
At the 2-year mark, overall survival rates were 72% with nivolumab/ipilimumab given first (with targeted therapy at disease progression) and 52% with dabrafenib/trametinib given first (with immunotherapy at disease progression)—an observation that became clear after about 10 months of treatment. Immunotherapy first also led to more durable responses, the study found.
Optimal Sequencing: An Unanswered Question
About half of all patients with metastatic melanoma have BRAF V600 mutations. Treatment with both BRAF/MEK inhibitors and nivolumab/ipilimumab has yielded survival improvements in this population but in different ways. The data have suggested that BRAF/MEK inhibitors “tend to splay the overall survival curve, whereas immunotherapy tends to raise the tail of the curve,” according to Dr. Atkins.
“The questions in 2015 [when DREAMseq was initiated] were which approach is preferred and, given that most patients would have access to both approaches, whether there is an optimal sequence,” he explained.
DREAMseq Details
The DREAMseq study enrolled 265 patients of an anticipated 300 patients (median age, 61 years; 63% male) with treatment-naive BRAF V600–mutant metastatic melanoma. Patients were randomly assigned to step 1 treatment with nivolumab/trametinib immunotherapy (arm A) or dabrafenib/trametinib targeted therapy (arm B). For arm A, patients received 12 weeks of induction combination immunotherapy and then nivolumab monotherapy for up to 72 weeks. For arm B, dabrafenib/trametinib was administered continuously.
Upon disease progression, patients were enrolled in step 2, which consisted of treatment with the alternate combination. Arm A switched over to dabrafenib/trametinib (arm C), and arm B switched over to nivolumab/ipilimumab (arm D). The primary endpoint of the trial was 2-year overall survival.
Response Rates
Initial overall response rates were 46% with nivolumab/ipilimumab and 43% with dabrafenib/trametinib. At data cutoff, 88% of the immunotherapy-first responders remained in response, as compared with 49% of the targeted therapy–first group, yielding a median duration of response that was not reached in the immunotherapy-first group and was 12.7 months in the targeted therapy–first group (P < .001). After disease progression, response rates to second-line treatment were 48% with dabrafenib/trametinib and 30% with nivolumab/ipilimumab.
Dr. Atkins noted: “Response rates were similar for the two step 1 regimens and for dabrafenib/trametinib whether used in step 1 or step 2. In contrast, nivolumab/ipilimumab appeared to be less effective after disease progression on dabrafenib/trametinib than as first-line therapy.”
Progression-Free and Overall Survival: Biphasic Pattern
“The progression-free and overall survival curves were biphasic, with curves crossing at 6 months and 10 months, respectively,” Dr. Atkins noted. “Nivolumab/ipilimumab yielded an increasing benefit in progression-free survival out to the 2-year time point.”
At a median follow-up of 27.7 months, progression-free survival showed a trend (P = .054) favoring patients who started on immunotherapy first. As for overall survival, of the 100 deaths in the trial, 38 occurred in patients who started on nivolumab/ipilimumab first and 62 occurred in those who started on targeted therapy, yielding a 20% difference in survival 20% (P = .0095; Table 1) at the 2-year time point. For all subsets, 2-year overall survival was better with nivolumab/ipilimumab first, he commented.
“The 95% repeated confidence interval around this 20% difference in overall survival ranged from 3% to 38%; the O’Brien-Fleming boundary was crossed based on this estimate. Although 59% of the information for the protocol-specified endpoint had not been met, the data safety and monitoring committee believed there was a clinically meaningful difference in overall survival. It recommended the study be closed to accrual and patients on first-line dabrafenib/trametinib be given the option to switch to nivolumab/ipilimumab, without the need for disease progression,” Dr. Atkins said.
A total of 24 patients died after less than 10 months on nivolumab/ipilimumab. These patients tended to have a worse prognosis and/or more adverse events and received “limited treatment” that did not include dabrafenib or trametinib upon disease progression. “This suggests that in addition to bad disease biology, the study criteria for crossover eligibility may have been too strict for optimal drug exposure and efficacy,” he added.
“Of note, even in the group where dabrafenib/trametinib is reported to do best—those with a good performance status, normal lactate dehydrogenase levels, and better disease stage, perhaps as a surrogate for fewer than three sites of disease—starting with nivolumab/ipilimumab showed a strong trend for improved 2-year overall survival (P = .054),” Dr. Atkins reported.
Toxicity Profile
For safety, grade ≥ 3 toxicity was higher with the immunotherapy-first strategy, at 60% vs 52% with the targeted therapy–first approach. There were three treatment-related deaths among patients who started on immunotherapy: two during the nivolumab/ipilimumab phase and one after switching to the targeted combination.
DISCLOSURE: Dr. Atkins reported relationships with Bristol Myers Squibb, Adagene, Agenus, Amgen, Apexigen, Arrowhead, Asher Bio, AstraZeneca, Aveo, Calithera, COTA, Eisai, Exelixis, Elpis, Fathom, Genentech-Roche, Idera, Immunocore, Iovance, Leads, Merck, Neoleukin, Novartis, Pfizer, Pneuma, Pyxis Oncology, PACT, Sanofi, Seagen, Scholar Rock, Simcha, Surface, Takeda, Valo Health, Werewolf, and X4 Pharmaceuticals. Dr. Schuchter reported personal or institutional relationships with Bristol Myers Squibb, Incyte, GlaxoSmithKline, Merck, and Pfizer.
REFERENCE
1. Atkins MB, Lee SJ, Chmielowski B, et al: DREAMseq: A phase III trial—ECOG-ACRIN EA6134. ASCO Plenary Series. Abstract 356154. Presented November 16, 2021.

