
Cathy Eng, MD

Van K. Morris, MD
A new ASCO guideline on the management of advanced colorectal cancer summarizes the latest treatments supported by quality data that could expand oncologists’ armamentarium and potentially improve survival outcomes.1
“[Colorectal cancer] remains the second-leading class of cancer deaths among men and women in the United States combined and is still a major issue globally,” said Co-Chair of the Guideline Expert Panel, Cathy Eng, MD, of Vanderbilt-Ingram Cancer Center, Nashville. “The reality is a majority of patients will present with a more advanced stage, and not all of those are surgically resectable, so it’s important that providers offer the best options available.”
Practice-Changing Recommendations
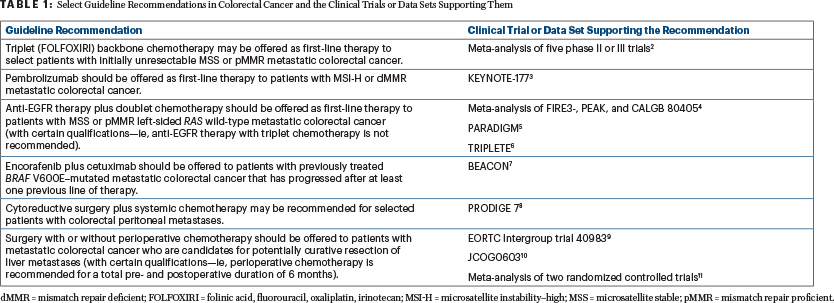
Although metastatic colorectal cancer often has a poor prognosis, clinical advances have emerged over the past decade that could translate into additional treatment options and reduced mortality rates. The Expert Panel attempted to incorporate such findings into the guideline when warranted by the quality of the evidence (Table 1).2-11
“One of the exciting developments we have seen is the use of immunotherapy for patients with microsatellite instability–high metastatic [colorectal cancer], with the phase III KEYNOTE-177 trial showing immunotherapy improved progression-free survival relative to the previously held standard of care—cytotoxic chemotherapy,” said Co-Chair of the Guideline Expert Panel, Van K. Morris, MD, of The University of Texas MD Anderson Cancer Center, Houston. “That’s certainly practice-changing for that subset of patients.”3
Therefore, based on these data, the guideline recommends offering pembrolizumab in the first-line setting to patients with microsatellite instability–high metastatic colorectal cancer.
Another discovery reflected in the guideline that spurred the new recommendations is from the BEACON study, which showed improved survival with targeted therapy with encorafenib plus cetuximab for BRAF V600E–mutated colorectal cancer—an aggressive subset that comprises approximately 8% to 15% of all patients with metastatic disease.12-15 The guideline recommends using encorafenib plus cetuximab for patients with previously treated BRAF V600E–mutant metastatic colorectal cancer that has progressed after at least one previous line of therapy.
“What we are seeing is, in certain subsets of patients with [colorectal cancer], we are able to move away from cytotoxic chemotherapy and toward more tailored, more effective, and potentially less-toxic agents for patients,” Dr. Morris said.
The new guideline also includes recommendations on various therapy indications, the role of immunotherapy and targeted therapies in certain subpopulations with colorectal cancer (eg, patients with RAS wild-type metastatic colorectal cancer), and the use of doublet vs triplet chemotherapy.
The guideline does not address all possible treatment options for colorectal cancer and instead is concentrated on what the panel thought would help clinical oncologists the most in terms of addressing unmet needs in patients with advanced colorectal cancer. The panel prioritized the latest data, ensuring the recommendations included data from the 2022 ASCO Annual Meeting, which occurred a few months before the document was finalized.
“We wanted to make sure people understood and felt comfortable with triplet therapy as a chemotherapy backbone for some of our patients,” Dr. Eng said. “Although prior studies have demonstrated its efficacy, the TRIPLETE trial showed triplet therapy was not beneficial in combination with anti-EGFR therapy.16 That data just came out this summer. Clinicians may not have seen that data, so by updating the guideline with the most recent data available, the hope is that it will enhance patient safety and efficacy.”
Looking to the Future
In the process of creating the guideline, the panel encountered several areas where more robust evidence is needed but that could potentially be important to clinical care in the future. For instance, the use of circulating tumor DNA (ctDNA) requires more data in the metastatic setting to guide decision-making, especially as a tool to evaluate responses to systemic therapies based on ctDNA status. However, Dr. Morris noted that he anticipates future clinical trials will likely address questions such as how oncologists can use ctDNA to inform colorectal cancer treatment as well as whether ctDNA can be leveraged to identify treatment responders vs nonresponders. This information could potentially spare some patients from being exposed to unnecessary toxicities of treatments not likely to confer a benefit.
“We also brought up the controversial data of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy, but the strength of the data was not present, and that’s important,” Dr. Eng added.
As these and other emerging areas of study continue to develop, Dr. Eng and Dr. Morris are optimistic that the guideline will serve as an easily accessible and clinically useful resource. “As we understand more about the different subsets of patients with [colorectal cancer] and how the underlying biology that drives people’s disease may be different, new results have come out in the past several years that have helped to advance treatment and improve survival in some of these patients,” Dr. Morris said. “With treatment options evolving, we wanted to provide an update that is backed by evidence-based medicine for the latest treatment recommendations.”
REFERENCES
1. Morris VK, et al: Treatment of metastatic colorectal cancer: ASCO Guideline. J Clin Oncol. October 17, 2022 (early release online).
2. Cremolini C, et al: Individual patient data meta-analysis of FOLFOXIRI plus bevacizumab versus doublets plus bevacizumab as initial therapy of unresectable metastatic colorectal cancer. J Clin Oncol 38:3314-3324, 2020.
3. André T, et al: Pembrolizumab in microsatellite-instability–high advanced colorectal cancer. N Engl J Med 383:2207-2218, 2020.
4. Ciliberto D, et al: The best strategy for RAS wild-type metastatic colorectal cancer patients in first-line treatment: A classic and Bayesian meta-analysis. Crit Rev Oncol Hematol 125:69-77, 2018.
5. Muro K, et al: First-line panitumumab versus bevacizumab in combination with mFOLFOX6 for RAS wild-type metastatic colorectal cancer: PARADIGM trial results. ESMO World Congress on Gastrointestinal Cancer 2022. Abstract LBA O-10. Presented June 29, 2022.
6. Rossini D, et al: Upfront modified fluorouracil, leucovorin, oxaliplatin, and irinotecan plus panitumumab versus fluorouracil, leucovorin, and oxaliplatin plus panitumumab for patients with RAS/BRAF wild-type metastatic colorectal cancer: The Phase III TRIPLETE study by GONO. J Clin Oncol 40:2878-2888, 2022.
7. Tabernero J, et al: Encorafenib plus cetuximab as a new standard of care for previously treated BRAF V600E-mutant metastatic colorectal cancer: Updated survival results and subgroup analyses from the BEACON study. J Clin Oncol 39:273-284, 2021.
8. Quénet F, et al: Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7). Lancet Oncol 22:256-266, 2021.
9. Nordlinger B, et al: Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983). Lancet Oncol 14:1208-1215, 2013.
10. Kanemitsu Y, et al: Hepatectomy followed by mFOLFOX6 versus hepatectomy alone for liver-only metastatic colorectal cancer (JCOG0603). J Clin Oncol 39:3789-3799, 2001.
11. Mitry E, et al: Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: A pooled analysis of two randomized trials. J Clin Oncol 26:4906-4911, 2008.
12. Davies H, et al: Mutations of the BRAF gene in human cancer. Nature 417:949-954, 2002.
13. Loupakis F, et al: KRAS codon 61, 146 and BRAF mutations predict resistance to cetuximab plus irinotecan in KRAS codon 12 and 13 wild-type metastatic colorectal cancer. Br J Cancer 101:715-721, 2009.
14. Tie J, et al: Optimizing targeted therapeutic development: Analysis of a colorectal cancer patient population with the BRAF(V600E) mutation. Int J Cancer 128:2075-2084, 2011.
15. Clarke CN, Kopetz ES: BRAF mutant colorectal cancer as a distinct subset of colorectal cancer: Clinical characteristics, clinical behavior, and response to targeted therapies. J Gastrointest Oncol 6:660-667, 2015.
16. Cremolini C, et al: Modified FOLFOXIRI plus panitumumab (mFOLFOXIRI/PAN) versus mFOLFOX6/PAN as initial treatment of patients with unresectable RAS and BRAF wild-type metastatic colorectal cancer (mCRC): Results of the phase III randomized TRIPLETE study by GONO. 2022 ASCO Annual Meeting. Abstract LBA3505. Presented June 4, 2022.
Originally published in ASCO Daily News. © American Society of Clinical Oncology. ASCO Daily News, October 19, 2022. All rights reserved.

