 Seminal research in the treatment of HER2-positive breast cancer has been led by Edith A. Perez, MD, the Serene M. and Frances C. Durling Professor of Medicine at the Mayo Clinic, Jacksonville, Florida. The ASCO Post asked Dr. Perez to share her approach to HER2-directed therapy.
Seminal research in the treatment of HER2-positive breast cancer has been led by Edith A. Perez, MD, the Serene M. and Frances C. Durling Professor of Medicine at the Mayo Clinic, Jacksonville, Florida. The ASCO Post asked Dr. Perez to share her approach to HER2-directed therapy.
Testing Considerations
Which test do you rely on to determine HER2 status and to select patients for anti-HER2 treatment?
My group recently looked for associations between certain tumor characteristics—HER2 protein expression, HER2 gene and chromosome 17 copy number, hormone receptor status—and disease-free survival in the NCCTG N9831 adjuvant trastuzumab (Herceptin) population.1 We confirmed that either immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH) testing is appropriate for patient selection for trastuzumab. You don’t need both tests if one is positive. We also found that trastuzumab benefit seems independent of HER2/centromere 17 ratio and chromosome 17 copy number.
There has been confusion regarding the recommendation from ASCO and the College of American Pathologists against withholding anti-HER2 treatment in patients with equivocal HER2 test results falling within ranges that qualified patients for the first adjuvant HER2-targeted trials (ie, the FDA guidelines). Research from our group can add to this discussion. We will soon be publishing data from N9831, showing the importance of using an IHC > 10% or a FISH ratio ≥ 2.0 (not > 2.2) as the relevant cutoffs for adjuvant anti-HER2 therapy.2,3
When do you retest patients for HER2 expression?
We know there are patients who are IHC0 or IHC1+ but have some HER2 gene amplification, and some may benefit from anti-HER2 treatment. We need to determine who they are. Expanded reflex testing, in which we retest patients whose tumors are IHC0, IHC1+, or FISH-negative (using the opposite test), can help do this.
We performed an analysis of expanded reflex testing and estimated that 3% of retested patients would be HER2-positive.4 We also found the cost-effectiveness ratio of expanded reflex testing in the adjuvant setting to be below commonly cited thresholds. This strategy could reduce the number of inappropriately classified tumors and identify an additional 4,700 patients per year who might benefit from adjuvant trastuzumab.
Are there other tumor markers that should be considered in making decisions about anti-HER2 therapy?
We know that estrogen receptor (ER)-positive tumors are associated with better outcomes in the setting of HER2-positivity, so ER status is relevant. But the degree of gene amplification, c-myc, polysomy 17, insulin growth factor receptor, and PTEN do not appear to be predictive of a benefit from adjuvant trastuzumab.
Treatment Regimens
 What do you consider to be the optimal adjuvant anti-HER2 regimen?
What do you consider to be the optimal adjuvant anti-HER2 regimen?
In deciding upon a chemotherapy backbone, I consider the patient’s overall condition. My first choice is usually an anthracycline-based regimen, due to the totality of the data from multiple trials (NCCTG N9831, NSABP B-31, HERA, FinHER, PACS04). I consider a non–anthracycline-based regimen, ie, TCH (docetaxel/carboplatin/trastuzumab), as a second-best alternative, based on data from BCIRG 006.
Do you favor sequential or concurrent use of trastuzumab with chemotherapy, and how long should trastuzumab be given?
N9831 is comparing trastuzumab given for 52 weeks following 12 weeks of paclitaxel (sequential treatment) to concurrent trastuzumab plus paclitaxel. We are seeing that the “hazard of disease events” (recurrences, contralateral breast cancer, second primaries, or death) is much lower, at least numerically, with the concurrent approach.
Disease-free survival at 5 years is 84.2% with concurrent treatment and 79.8% with sequential treatment. Giving trastuzumab concurrently, therefore, should produce better outcomes. One year of trastuzumab therapy is still recommended, but shorter and longer durations of treatment are currently being studied.
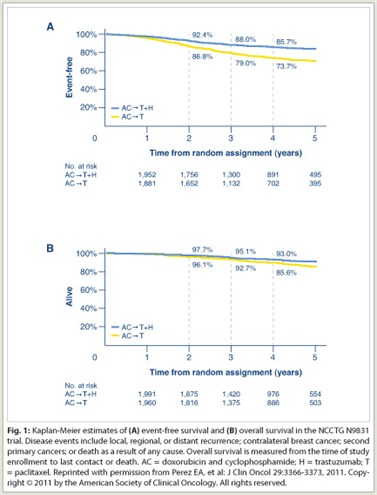 It is gratifying to see the long-term benefit of adjuvant trastuzumab emerging. We recently published our 4-year follow-up of the joint analysis of N9831 and B-31 (see sidebar on page 8), which showed 5-year disease-free survival to be 85.7% for AC→T+H (doxorubicin/cyclophosphamide, followed by paclitaxel plus trastuzumab), vs 73.7% for AC→T, and overall survival rates of 93% vs 85.6%, respectively (see Fig. 1 on page 3). The curves continue to diverge, showing the long-term impact of anti-HER2 therapy, but at the same time we are not curing all our patients. We still have work to do.
It is gratifying to see the long-term benefit of adjuvant trastuzumab emerging. We recently published our 4-year follow-up of the joint analysis of N9831 and B-31 (see sidebar on page 8), which showed 5-year disease-free survival to be 85.7% for AC→T+H (doxorubicin/cyclophosphamide, followed by paclitaxel plus trastuzumab), vs 73.7% for AC→T, and overall survival rates of 93% vs 85.6%, respectively (see Fig. 1 on page 3). The curves continue to diverge, showing the long-term impact of anti-HER2 therapy, but at the same time we are not curing all our patients. We still have work to do.
What are your recommendations for managing metastatic disease?
I recommend rebiopsying the tumor to assay ER and HER2 status. And I use combinations in most patients, as opposed to single-agent trastuzumab or lapatinib (Tykerb), which are associated with insufficient response rates. These regimens are often taxane-based (such as weekly paclitaxel/carboplatin). Other options are trastuzumab, vinorelbine plus trastuzumab, and aromatase inhibitors plus lapatinib or trastuzumab.
For refractory metastatic disease, the essence of management is to continue the anti-HER2 therapy. Good options include capecitabine (Xeloda) plus trastuzumab or lapatinib, trastuzumab plus lapatinib, vinorelbine plus trastuzumab, and others.
Looking Ahead
What does the future hold for HER2-directed therapy?
There is a strong push to identify specific molecular markers and further define the signaling pathways in HER2 disease. We are also looking for immune correlates and multigene signatures using next-generation sequencing. We are validating assays for P95, extracellular cleaved HER2 that lacks the trastuzumab-binding domain and thus may be a source of trastuzumab resistance.
For treatment in the adjuvant setting, we are seeing encouraging data for pertuzumab, and in the metastatic setting, the novel T-DM1 drug-antibody conjugate is exciting. In a phase II trial, T-DM1 was associated with a 41% reduction in disease progression, compared with standard care, and a median disease-free survival exceeding 14 months.5 It is being studied in the first-line setting in the large (n = 1,092) phase III MARIANNE trial in combination with pertuzumab and as a single agent. And the EMILIA trial is comparing T-DM1 to lapatinib/capecitabine in refractory patients.
Another fascinating compound is GRN1005, a peptide-drug conjugate that is designed to enable paclitaxel to penetrate the intracerebral compartment via the LRP-1 receptor on the blood-brain barrier. The hope is that this would be an effective treatment of brain metastasis, which is clearly an area of unmet need, especially in HER2-positive patients. ■
Disclosure: Dr. Perez has received research funding from Genentech and GlaxoSmithKline.
SIDEBAR: Adding Trastuzumab to Adjuvant Chemotherapy
References
1. Perez EA, Reinholz MM, Hillman DW, et al: HER2 and chromosome 17 effect on patient outcome in the N9831 adjuvant trastuzumab trial. J Clin Oncol. 2010;28(28):4307-4315.
2. Perez EA, Suman VJ, Davidson NE, et al: Sequential versus concurrent trastuzumab in adjuvant chemotherapy for breast cancer. J Clin Oncol. October 31, 2011 (early release online).
3. Perez EA, Dueck AC, McCullough A, et al: Do the ASCO/CAP 2007 HER2 Testing Guidelines accurately identify patients who will benefit from adjuvant trastuzumab? Data from the North Central Cancer Treatment Group N9831 Trial. J Natl Cancer Inst. In press
4. Garrison LP, Lalla D, Brammer M, et al: Assessing the potential cost-effectiveness of retesting IHC0, IHC1+ or FISH-negative early-stage breast cancer patients for HER2 status. 2011 ASCO Annual Meeting. Abstract 6133. Presented June 4, 2011.
5. Hurvitz S, Dirix L, Kocksis J, et al: Trastuzumab emtansine (T-DM1) versus trastuzumab plus docetaxel in previously untreated HER2-positive metastatic breast cancer: primary results of a randomized multicenter open-label phase II study. 2011 European Multidisciplinary Cancer Congress. Abstract 5001. Presented September 25, 2011.

