In newly diagnosed advanced ovarian cancer lacking a BRCA mutation, the addition of durvalumab and olaparib to standard therapy significantly improved progression-free survival, in a planned interim analysis of the international phase III DUO-O trial presented at the 2023 ASCO Annual Meeting1 and at a press briefing prior to the presentation.
DUO-O evaluated the benefit of incorporating both the PD-L1 antibody durvalumab and the PARP inhibitor olaparib to standard treatment with paclitaxel/carboplatin and bevacizumab. Durvalumab was incorporated upfront, and both durvalumab and olaparib were added to bevacizumab maintenance. The population included 1,130 patients with newly diagnosed non–BRCA-mutated advanced ovarian cancer.
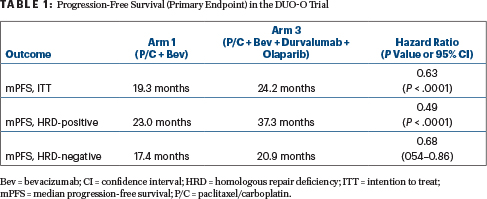
The study met its co-primary endpoints: the addition of durvalumab and olaparib resulted in a 37% reduction in the risk of disease progression in the intention-to-treat analysis (P < .0001) and a 51% reduction among patients whose tumors had homologous recombination deficiency (HRD; P < .0001).

Philipp Harter, MD, PhD

Merry Jennifer Markham, MD
Presenting author and co-investigator Philipp Harter, MD, PhD, Director of the Department of Gynecology and Gynecologic Oncology at the Evangelische Kliniken Essen-Mitte hospital in Essen, Germany, described the benefit seen with the novel regimen as both “statistically significant and clinically meaningful.”
“Progress has not been quick enough for our patients with advanced ovarian cancer. This is a huge step forward,” according to ASCO expert Merry Jennifer Markham, MD, Chief of the Division of Hematology and Oncology at the University of Florida Health Cancer Center in Gainesville. “It’s important to note that the benefit was seen across the board. To be able to have options for all comers [with or without BRCA mutation] is very meaningful.”
As the investigators noted, phase III trials have not yet shown benefit for the addition of checkpoint inhibitors to standard therapy in this population. However, in the phase II MEDIOLA study, a regimen containing durvalumab, bevacizumab, and olaparib showed promising activity in patients with platinum-sensitive disease and no germline BRCA mutation.2 DUO-O is the first trial to show an effect of a PARP inhibitor combined with an immune checkpoint inhibitor.
About the Study
The international phase III DUO-O/ENGOT-ov46/AGO-OVAR 23/GOG-3025 trial enrolled 1,130 patients with stage III or IV high-grade epithelial tumors lacking somatic BRCA mutations. Patients were either HRD-positive (39%), HRD-negative (55%), or HRD-unknown (6%). All had completed or were planned to receive debulking surgery.
Patients were randomly assigned to one of three arms, all of which initiated therapy with upfront paclitaxel/carboplatin plus bevacizumab, followed by maintenance bevacizumab. For patients in arms 2 and 3, durvalumab was added to both the upfront and maintenance regimens. Arm 3 was similar to arm 2, except that olaparib was added to the maintenance regimen. Specifically, the regimens were:
- Arm 1: standard treatment with upfront paclitaxel/carboplatin plus bevacizumab, followed by maintenance bevacizumab for 15 months
- Arm 2: standard treatment as above, plus durvalumab upfront and durvalumab added to bevacizumab as maintenance for 24 months
- Arm 3: standard treatment as above, plus durvalumab upfront with both durvalumab and olaparib added to bevacizumab as maintenance for 24 months.
At the press briefing, co-principal investigator Carol Aghajanian, MD, Chief of the Gynecologic Medical Oncology Service at Memorial Sloan Kettering Cancer Center, New York, emphasized that the trial was restricted

Carol Aghajanian, MD
to patients with non–BRCA-mutated tumors. “Previous phase III trials have focused solely on the BRCA-mutated group or a combination of BRCA-mutated and non–BRCA-mutated disease. And unlike previous studies of maintenance PARP inhibitors, DUO-O permitted patients with stable disease as well as responders to receive maintenance therapy,” she said.
Findings of the First Planned Interim Analysis
The investigators reported the following results for progression-free survival (Table 1):
- An increase in progression-free survival for patients in arm 3 (durvalumab/olaparib) compared with arm 1: median of 24.2 months vs 19.3 months
- A benefit for durvalumab/olaparib in all preplanned subgroups
- Longest median progression-free survival in HRD-positive patients: median of 37.3 months with durvalumab/olaparib
- Benefit for durvalumab/olaparib in HRD-negative patients as well: median 20.9 months
- No significant difference between arm 3 vs arm 2; both contained durvalumab, but arm 3 contained olaparib for maintenance.
“We have seen a consistent benefit in all subgroups we looked at, regarding time and outcome of cytoreductive surgery, geographic region, age, performance status, stage of disease at diagnosis, HRD status, and PD-L1 expression,” Dr. Harter reported.
A numerical improvement in progression-free survival was observed in arm 2 vs arm 1, but statistical significance has not yet been reached (hazard ratio = 0.87). This endpoint will be reassessed at the time of the final progression-free survival analysis, he said.
Approximately 90% of patients completed the trial regimens. Serious adverse events were reported in 34% of patients in arm 1, 43% of arm 2, and 39% of arm 3. The most common grade ≥ 3 adverse events were neutropenia (26% of arm 1; 28% of arm 2; 31% of arm 3) followed by anemia (8%, 8%, and 24%, respectively). Dose modifications were required in 72%, 80%, and 85%, and treatment was discontinued by 20%, 26%, and 35%, respectively.
“The safety and tolerability of these combinations are broadly consistent with that observed in prior clinical trials and the known profiles of the individual medicines,” Dr. Harter observed.
What Does Durvalumab Contribute?
During the discussion and at the press briefing, questions were raised regarding the relative contribution of durvalumab, since DUO-O lacked a control arm in which the maintenance regimen included the PARP inhibitor. Dr. Harter explained the study was initiated before two phase III trials showed the benefit of maintenance with olaparib.3,4 “The standard of care changed during the recruitment of the trial participants. We had already recruited around 300 patients and could not change the design of the study” to reflect the importance of olaparib, he said.
KEY POINTS
- In women with newly diagnosed non–BRCA-mutated advanced ovarian cancer, DUO-O evaluated the benefit of adding both durvalumab and olaparib to standard treatment with paclitaxel/carboplatin and bevacizumab.
- Durvalumab was incorporated upfront, and both durvalumab and olaparib were added to bevacizumab maintenance.
- The novel regimen resulted in a 37% reduction in the risk of disease progression in the intention-to-treat analysis (P < .0001) and a 51% reduction among patients whose tumors had homologous recombination deficiency (HRD; P < .0001).
Dr. Aghajanian said investigators could not comment on the contributions of durvalumab to the outcomes, but preclinical studies have suggested there may be synergy between durvalumab and olaparib. “Certainly, if we had it to do over again [ie, if the study were designed today], we would have a fourth arm that had bevacizumab and olaparib maintenance so we could do a comparison.”
DISCLOSURE: Dr. Harter has received honoraria or institutional research funding from AstraZeneca, Clovis Oncology, Eisai, GlaxoSmithKline, Eli Lilly, MSD Oncology, Roche, Sotio, Stryker, Zai Lab, Immunogen, and Genmab; and has served as a consultant or advisor to AstraZeneca, Clovis Oncology, GlaxoSmithKline, Immunogen, Merck, Roche, and Tesaro. Dr. Markham reported financial relationships with Pfizer and GlaxoSmithKline. Dr. Aghajanian has received institutional research funding from AbbVie, AstraZeneca, Clovis Oncology, and Genentech/Roche; and has served as a consultant or advisor to AstraZeneca/Merck, Eisai, and Repare Therapeutics.
REFERENCES
1. Harter P, Trillisch F, Okamoto A, et al: Durvalumab with paclitaxel/carboplatin and bevacizumab followed by maintenance durvalumab, bevacizumab and olaparib in patients with newly diagnosed advanced ovarian cancer without a tumor BRCA1/BRCA2 mutation: Results from the randomized, placebo-controlled phase III DUO-O/ENGOT-ov46/AGO-OVAR 23/GOG-3025 trial. 2023 ASCO Annual Meeting. Abstract LBA5506. Presented June 3, 2023.
2. Banerjee S, Imbimbo M, Roxburgh P, et al: Phase II study of olaparib plus durvalumab with or without bevacizumab (MEDIOLA): Final analysis of overall survival in patients with non-germline BRCA-mutated platinum-sensitive relapsed ovarian cancer. Ann Oncol 33(suppl 7):S788-S789, 2022.
3. Ray-Coquard I, Pautier P, Pignata S, et al: Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med 381:2416-2428, 2019.
4. Moore K, Colombo N, Scambia G, et al: Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 379:2495-2505, 2018.


