Updates of landmark trials of immune checkpoint inhibitors as first-line therapy for advanced HER2-negative esophagogastric cancers were presented at the 2022 ASCO Gastrointestinal Cancers Symposium. The studies upheld previous findings of an overall survival benefit when these agents are paired with chemotherapy and an enrichment of benefit by PD-L1 expression.
CheckMate 649 evaluated nivolumab plus chemotherapy vs chemotherapy,1 whereas KEYNOTE-590 compared pembrolizumab plus chemotherapy vs chemotherapy in previously untreated patients.2 Together, these studies ushered in immunotherapy as a first-line option for esophagogastric cancers. However, the data were less encouraging in KEYNOTE-062, which found no superiority for pembrolizumab, with or without chemotherapy, over chemotherapy alone in patients with a PD-L1 combined positive score (CPS) ≥ 1.3
At the meeting, investigators reported longer-term data, including efficacy according to PD-L1 CPS enrichment. They also reported no new safety signals for any of the three trials.
CheckMate 649 Update

Kohei Shitara, MD
Kohei Shitara, MD, of the National Cancer Center Hospital East, Kashiwa, Japan, presented updated findings for nivolumab plus chemotherapy vs chemotherapy in CheckMate 649. This study randomly assigned 1,581 patients to nivolumab plus FOLFOX (fluorouracil/leucovorin/oxaliplatin) or XELOX (capecitabine/oxaliplatin) or FOLFOX or XELOX alone.1 The population included patients with HER2-negative or unknown gastric cancer, gastroesophageal junction cancer, and esophageal adenocarcinoma.
In the analysis after 12 months of minimum follow-up, nivolumab plus chemotherapy significantly improved overall and progression-free survival.4 “These clinically meaningful improvements were maintained with longer follow-up,” Dr. Shitara reported.
In an expanded analysis after 24 months of follow-up, median overall survival was 13.8 months with nivolumab plus chemotherapy vs 11.6 months with chemotherapy (hazard ratio [HR] = 0.79; 95% confidence interval [CI] = 0.71–0.88); 28% and 19% of patients, respectively, were alive. “The overall survival benefit was seen across key subgroups and was enriched at higher PD-L1 CPS cutoffs,” he said. For patients with a CPS ≥ 5, median overall survival was 14.4 months with nivolumab plus chemotherapy and 11.1 months with chemotherapy alone (HR = 0.69).
Median progression-free survival was 7.7 months with nivolumab plus chemotherapy vs 6.9 months with chemotherapy alone (HR = 0.79; 95% CI = 0.70–0.89). At 24 months, 16% and 10%, respectively, were progression-free.
The combination also resulted in higher objective response rates across all PD-L1 CPS subgroups and more deep and durable responses regardless of PD-L1 CPS ≥ 5 or < 5. Treatment with nivolumab plus chemotherapy resulted in more than 80% reduction in tumor burden in 27% of the PD-L1 CPS ≥ 5 population and in 19% of the CPS < 5 subset. With chemotherapy, these percentages were 18% and 13%, respectively.
Responses to nivolumab plus chemotherapy were observed in 60% of the CPS ≥ 5 population and 55% of the CPS < 5 subset, vs 45% to 46% of both CPS subsets receiving chemotherapy. The median duration of response was 9.7 and 7.7 months, respectively, for those CPS subsets and was less than 7 months with chemotherapy.
“Overall survival and objective response benefit across PD-L1 CPS subgroups was consistent with the all-randomized population when excluding patients with [microsatellite instability–high] tumors,” he said.
CheckMate 649 investigators also analyzed progression-free survival from randomization to disease progression after subsequent therapy, which was received by 41% of the nivolumab-plus-chemotherapy arm and 44% of the chemotherapy-alone arm. They found nivolumab plus chemotherapy was also favored, with a median time to second disease progression of 12.2 months vs 10.4 months (HR = 0.75; 95% CI = 0.67–0.84).
KEYNOTE-590 Update
Jean-Philippe Metges, MD, of the Institut de Cancerologie et d’Hematologie ARPEGO Network, Brest, France, presented updated data from KEYNOTE-590.2 The investigators of this trial previously reported a significant overall survival and progression-free survival benefit with pembrolizumab plus chemotherapy vs chemotherapy as a first-line treatment in 749 patients with advanced or metastatic esophageal or gastroesophageal junction cancer.2 About half had a PD-L1 CPS ≥ 10.5
At the first interim analysis, median overall survival was 12.4 months vs 9.8 months (HR = 0.73; P < .0001), and median progression-free survival was 6.3 months vs 5.8 months (P < .0001). After an additional 12 months of follow-up, pembrolizumab plus chemotherapy continued to show a meaningful improvement in overall survival and progression-free survival, especially in patients with a higher PD-L1 CPS (Table 1).
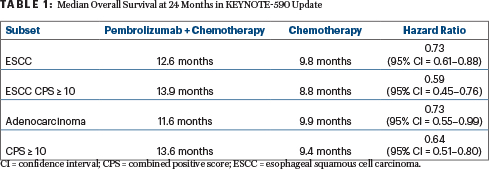
Median progression-free survival was 6.3 with pembrolizumab plus chemotherapy vs 5.8 months with chemotherapy alone (HR = 0.65) for patients with esophageal squamous cell carcinoma; 7.5 vs 5.5 months (HR = 0.51), respectively, for those with a PD-L1 CPS ≥ 10; and 6.3 vs 5.7 months (HR = 0.61), respectively, for patients with adenocarcinoma.
In all randomly assigned patients, the objective response rate was 45.0% vs 29.3%, and the median duration of response was 8.3 vs 6.9 months, with 20.4% and 6.2% of the arms, respectively, continuing to respond after at least 24 months. Change from baseline scores for pain, dysphagia, global health status, and quality of life was similar between the treatment arms.
“This is a confirmation. We have a new standard in patients with locally advanced and metastatic esophageal cancer, including gastroesophageal adenocarcinoma,” Dr. Metges commented.
KEYNOTE-062 Update

Zev A. Wainberg, MD
In contrast to the benefit shown for checkpoint inhibitors when paired with chemotherapy in the other two trials, pembrolizumab with or without chemotherapy did not improve survival over chemotherapy in KEYNOTE-062. The update from this trial was presented by Zev A. Wainberg, MD, of the University of California Los Angeles School of Medicine.3
KEYNOTE-062 enrolled 763 patients with locally advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma with a PD-L1 CPS ≥ 1 and no previous treatment. Dr. Wainberg presented new findings after an additional 25 months of follow-up and a median time from randomization of 54 months.
In the study’s primary analysis, pembrolizumab with and without chemotherapy was not superior in terms of overall survival (HR = 0.91) or progression-free survival (HR = 0.84) in patients with a CPS ≥ 1, but the study met the noninferiority statistical endpoint in patients with a CPS ≥ 10, and there were fewer adverse events in the pembrolizumab arms.6
“With 2 additional years of long-term follow-up, the findings are consistent with data from the final published analysis,” Dr. Wainberg said. “Pembrolizumab was noninferior to chemotherapy for overall survival in patients with PD-L1 CPS ≥ 1 tumors, which was particularly the case in patients with PD-L1 CPS ≥ 10 tumors (Table 2).”
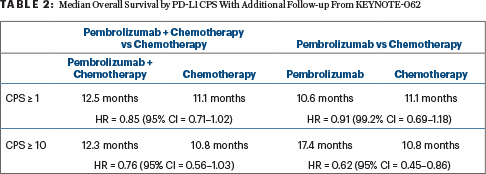
At 24 months, 24.5% of the pembrolizumab arm and 18.8% of the chemotherapy arm were alive; in the CPS ≥ 10 population, these rates were 28.3% and 21.2%, respectively.
Dr. Wainberg emphasized that the finding of noninferiority of single-agent pembrolizumab is not an endorsement for its use. “I am not suggesting by any means that we should consider pembrolizumab as a single agent in patients with metastatic microsatellite-stable gastric cancer. The statistical assessment of noninferiority is a different question. It doesn’t mean we should use that in practice, especially in light of other recent data sets,” he said.
Pembrolizumab plus chemotherapy will be further investigated in the first line setting in the phase III KEYNOTE-859 trial in more than 1,500 patients with advanced unresectable or metastatic gastric or gastroesophageal adenocarcinoma. The primary endpoint is overall survival.
DISCLOSURE: Dr. Shitara reported financial relationships with Bristol Myers Squibb, Takeda, AbbVie, Boehringer Ingelheim, Daiichi Sankyo, GlaxoSmithKline, Janssen, Lilly, MSD, Novartis, Ono Pharmaceutical, Pfizer, and Taiho Pharmaceutical. Dr. Metges reported financial relationships with Bristol Myers Squibb, Lilly, Novartis, Sanofi, Syncore, Amgen, LEO Pharma, and MSD Oncology. Dr. Wainberg reported financial relationships with Amgen, Array BioPharma, AstraZeneca/Medimmune, Bayer, Bristol Myers Squibb, Daiichi Sankyo/AstraZeneca, Five Prime Therapeutics, Ipsen, Lilly, MacroGenics, Merck, Merck KGaA, Novartis, and QED Therapeutics.
REFERENCES
1. Shitara K, Janjigian YY, Moehler MH, et al: Nivolumab plus chemotherapy versus chemotherapy as first-line treatment for advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma: Expanded efficacy, safety, and subgroup analyses from CheckMate 649. 2022 ASCO Gastrointestinal Cancers Symposium. Abstract 240. Presented January 20, 2022.
2. Metges JP, Kato K, Sun JM, et al: First-line pembrolizumab plus chemotherapy versus chemotherapy in advanced esophageal cancer: Longer-term efficacy, safety and quality-of-life results from the phase 3 KEYNOTE-590 study. 2022 ASCO Gastrointestinal Cancers Symposium. Abstract 241. Presented January 20, 2022.
3. Wainberg ZA, Shitara K, van Cutsem E, et al: Pembrolizumab with or without chemotherapy versus chemotherapy alone for patients with PD-L1–positive advanced gastric or gastroesophageal junction adenocarcinoma: Update from the phase 3 KEYNOTE-062 trial. 2022 ASCO Gastrointestinal Cancers Symposium. Abstract 243. Presented January 20, 2022.
4. Janjigian YY, Shitara K, Moehler M, et al: First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 398:27-40, 2021.
5. Sun JM, Shen L, Shah MA, et al: Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): A randomised, placebo-controlled, phase 3 study. Lancet 398:759-771, 2021.
6. Shitara K, Van Cutsem E, Bang YJ, et al: Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: The KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol 6:1571-1580, 2020.

