As in the years before the COVID-19 pandemic, the 2023 ASCO GI Cancers Symposium—its 20th such gathering—played to a full ballroom. It featured a stellar lineup of experts in the field as well as high-quality, impactful research in esophageal, gastric, hepatocellular, pancreatic, biliary tract, and colorectal cancers. In addition to our in-depth coverage of pivotal research from this conference, The ASCO Post briefly highlights the following studies of new treatments in gastrointestinal oncology.
Regorafenib in Refractory Advanced Gastroesophageal Cancer
The final results from the INTEGRATE-IIa trial in treatment-refractory advanced gastroesophageal cancer showed that treatment with regorafenib significantly improved overall survival, reducing the risk of death by 32% as compared with placebo, according to Nick Pavlakis, MBBS, PhD, of the University of Sydney, Australia.1

Nick Pavlakis, MBBS, PhD
“In the INTEGRATE-IIa study, regorafenib also delayed deterioration in quality of life and prolonged progression-free survival, with no new toxicity signals. The benefit was seen in all preplanned subgroups, offering a new treatment option in the refractory setting,” Dr. Pavlakis said.
After second-line treatment, patients with refractory advanced gastroesophageal cancer have few options. Based on a previous trial showing an improvement in progression-free survival with regorafenib,2 the global INTEGRATE-IIa study was initiated to determine its impact on overall survival in treatment-refractory patients. The 251 participants were randomly assigned to receive regorafenib at 160 mg daily or placebo, both with best supportive care.
Hazard ratios (HRs) favoring regorafenib were 0.68 (P = .006) for overall survival, with a 12-month survival rate of 19% vs 6% for placebo, and 0.53 for progression-free survival (P < .0001). Regorafenib also significantly delayed deterioration in global quality of life (P = .0043). A significant improvement in overall survival was observed in a pooled analysis of data from INTEGRATE and INTEGRATE-IIa (HR = 0.70; P = .001), Dr. Pavlakis reported.
Based on an earlier signal of a stronger subgroup effect in the Korean population, the investigators performed a subgroup analysis in INTEGRATE-IIa comparing outcomes in Asian vs non-Asian populations. Benefit in overall survival was observed with regorafenib treatment in all prespecified patient subgroups, with no region-specific subgroup effect observed.
HER-Vaxx in Gastric or Gastroesophageal Junction Cancer
In patients with HER2-positive advanced or metastatic gastric or gastroesophageal junction adenocarcinoma, immunization with a B-lymphocyte-stimulating, HER2-directed vaccine—HER-Vaxx (also known as IMU-131)—plus chemotherapy yielded deep and durable responses and significantly better overall survival compared with chemotherapy alone—14.0 vs 8.3 months, respectively (HR = 0.558; P = .054 [one-sided significance < .10]), according to Tanuj Chawla, MD, of Tata Medical Center, Kolkata, India.3
The design of the phase II open-label HER-Vaxx HERIZON study was based on the phase III open-label ToGA study, which found a survival benefit for trastuzumab plus chemotherapy in patients with HER2-positive advanced gastric or gastroesophageal junction cancer.4 In the HER-Vaxx HERIZON study, conducted in India and Eastern Europe, where trastuzumab is not easily available, the investigators hoped to obtain similar responses by adding HER-Vaxx to chemotherapy.

“Vaccination with HER-Vaxx induced persistent HER2-specific antibodies that correlated with clinical response as proof of concept for a first-in-class B-cell immunotherapy based on HER2 peptides.”— Tanuj Chawla, MD
Tweet this quote
Median progression-free survival was 6.9 months with HER-Vaxx plus chemotherapy vs 6.0 months with chemotherapy. The median duration of response was 30 weeks vs 19 weeks, respectively, and safety profiles of the arms were similar.
The study enrolled 36 patients with HER2-overexpressing advanced or metastatic disease not previously treated with an -anti-HER2 agent. Patients were randomly assigned to HER-Vaxx plus chemotherapy or chemotherapy alone. The experimental arm received a 50-microgram dose of HER-Vaxx, injected intramuscularly on days 0, 14, 35, 77, and then every 9 weeks until disease progression. Cisplatin plus either fluorouracil or capecitabine, or oxaliplatin plus capecitabine, was the standard chemotherapy regimen administered to participants in both groups beginning on day 0 and then every 21 days (maximum of six cycles or disease progression). The primary endpoint was overall survival.
“Vaccination with HER-Vaxx induced persistent HER2-specific antibodies that correlated with clinical response as proof of concept for a first-in-class B-cell immunotherapy based on HER2 peptides,” Dr. Chawla reported.
Now underway is the phase II nextHERIZON study (ClinicalTrials.gov identifier NCT05311176). Its two noncomparative arms are evaluating HER-Vaxx plus chemotherapy (ramucirumab plus paclitaxel) and HER-Vaxx plus pembrolizumab in patients with metastatic HER2-overexpressing gastric or gastroesophageal junction cancer who have experienced disease progression after treatment with trastuzumab. An additional phase II study in the neoadjuvant setting, neoHERIZON, is also planned in HER2-positive gastric cancer.
‘Negative Hyperselection’ of Wild-Type Tumors May Aid Treatment Selection
A biomarker analysis from the PARADIGM trial in metastatic colorectal cancer, based on circulating tumor DNA (ctDNA), has shown that the presence or absence of gene alterations may be as important as tumor sidedness in selecting treatment with an inhibitor of the epidermal growth factor receptor (EGFR), such as panitumumab, in metastatic colorectal cancer.5
In patients without gene alterations (ie, the “hyperselected” population), overall survival was longer with panitumumab than with bevacizumab, regardless of the primary tumor’s sidedness. In patients harboring any gene alteration, on the other hand, panitumumab was similar or inferior to bevacizumab, irrespective of sidedness, according to Kohei Shitara, MD, of the National Cancer Center Hospital East, Kashiwa, Japan.

Kohei Shitara, MD
“Negative hyperselection using ctDNA rather than tumor sidedness may identify appropriate patients for first-line panitumumab over bevacizumab,” Dr. Shitara said. “Overall survival in the hyperselected population favored panitumumab over bevacizumab. In contrast, panitumumab was not better than bevacizumab in patients with gene alterations.”
PARADIGM was a phase III, open-label, multicenter trial in patients with RAS wild-type metastatic colorectal cancer that evaluated first-line leucovorin, fluorouracil, and oxaliplatin plus panitumumab or bevacizumab.6 It reported that in patients with left-sided primaries, panitumumab improved overall survival; median survival was 37.9 months with panitumumab vs 34.3 months with bevacizumab (HR = 0.82, P = .031). No difference was seen between the arms in patients with right-sided tumors (HR = 1.09).
The biomarker study aimed to reveal more about gene alterations in ctDNA and their relationship to anti-EGFR therapy resistance as a means of better selecting patients for anti-EGFR therapy. Plasma ctDNA samples were taken at baseline from 733 patients (91% of the full-analysis set), revealing at least one gene alteration in 204 patients (28%). These gene alterations included KRAS/NRAS (8%); BRAF V600E (11%); PTEN (5%); EGFR (3%); HER2 amplification (5%); MET amplification (1%); and RET, NTRK1, and ALK fusions (1%). Of note, right-sided tumors were considerably more likely to have BRAF, KRAS, and PTEN alterations than left-sided tumors. The exclusion of this gene-altered group resulted in 529 “hyperselected” patients (72%).
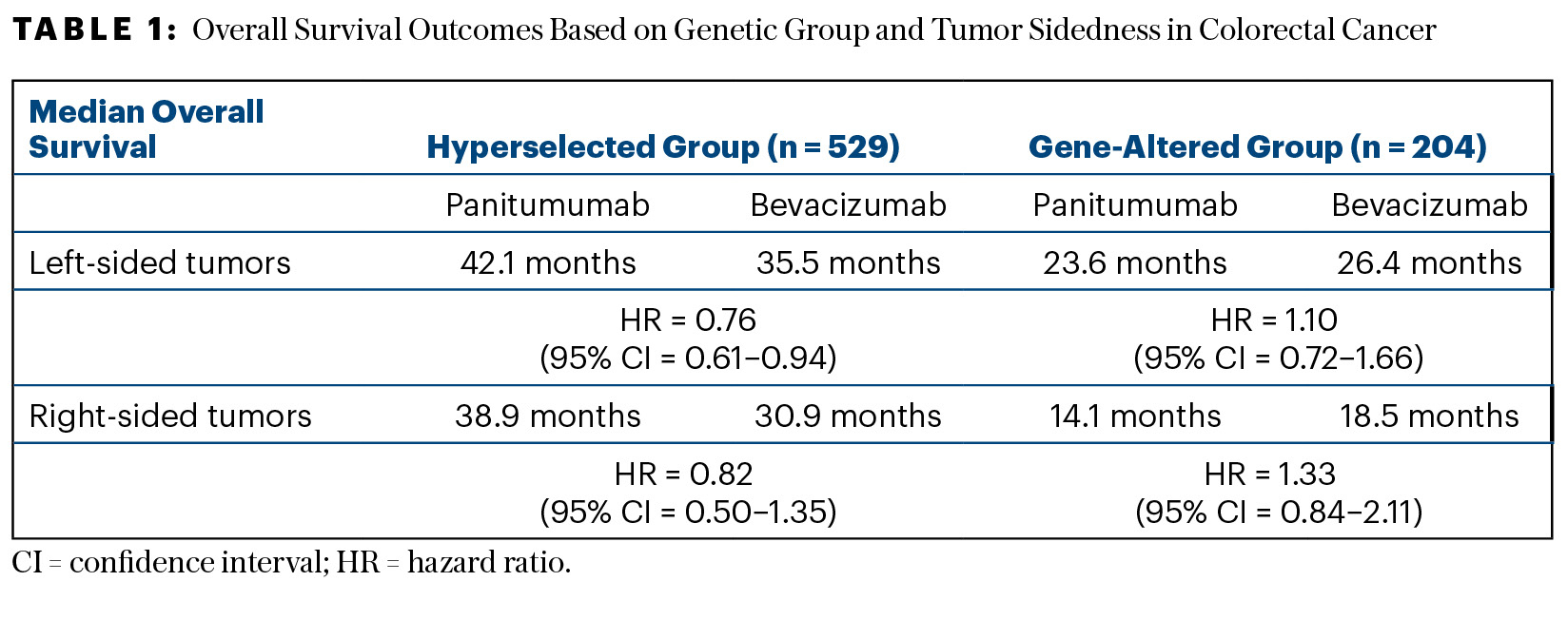
Survival outcomes were determined in the overall population according to the presence or absence of gene alterations. In the hyperselected (nonmutated) group, median overall survival was 41.3 months with panitumumab and 34.4 months with bevacizumab (HR = 0.75; 95% confidence interval [CI] = 0.62–0.92)—a gain of 7 months with the EGFR inhibitor. For the gene-altered group, panitumumab fared worse, yielding a median survival of 19.0 months vs 22.2 months with bevacizumab (HR = 1.14; 95% CI = 0.84–1.14). The P value for interaction was significant (P = .029), Dr. Shitara reported. Table 1 shows the overall survival outcomes according to genetic group and sidedness.
Similar patterns were shown in the progression-free survival analysis. The investigators hypothesized that sidedness is a surrogate for gene alterations. The exclusion of patients with these alterations may result in selection of patients who are good candidates for anti-EGFR therapy, such as panitumumab, he said.
SBRT Added to Sorafenib in Hepatocellular Carcinoma
Patients with hepatocellular carcinoma treated with stereotactic body radiotherapy (SBRT) and sorafenib achieved improved overall and progression-free survival compared with sorafenib alone in the phase III NRG/RTOG 1112 study.7 Median overall survival was 15.8 months with SBRT plus sorafenib vs 12.3 months with sorafenib alone (HR = 0.77; one-sided P = .055), according to Laura Dawson, MD, FASTRO, FRCPC, of the Princess Margaret Cancer Centre in Toronto.
The 193 patients were randomly assigned to receive either sorafenib at 400 mg twice daily or SBRT (27.5–50 Gy in five fractions) followed by sorafenib at 200 mg twice daily, increased to 400 mg twice daily after 28 days. Radiation therapy was personalized with individualized doses. The median sum of the primary tumor diameter was 7.8 cm, and the volume was 180 cc. Three-quarters of the patients had macrovascular invasion. The study’s primary endpoint was overall survival.

Laura Dawson, MD, FASTRO, FRCPC
The standard of care for patients with hepatocellular carcinoma who are unsuitable for surgery, ablation, and/or transarterial chemoembolization was sorafenib at the time of the study’s inception. The standard of care changed with the results of the IMbrave150 trial,8 which found a survival advantage with atezolizumab plus bevacizumab compared with sorafenib, leading to early closure of NRG/RTOG 1112, with reduction in power. The role of radiation therapy in the management of this cancer has been unclear for decades; NRG/RTOG 1112 was designed to help define it, specifically to evaluate the role of SBRT plus systemic therapy in a phase III trial, Dr. Dawson said.
In addition to the survival benefit, patients receiving SBRT and sorafenib, vs sorafenib alone, had a median progression-free survival of 9.2 months and 5.5 months, respectively (HR = 0.55; P = .0001), with estimated 12-month progression-free survival rates of 37% and 20%, respectively. The addition of SBRT also delayed the time to disease progression, with a median of 18.5 months vs 9.5 months, respectively (HR = 0.69; P = .034).
The overall survival benefit remained significant in a prespecified multivariable analysis (HR = 0.72, P = .042). There were no major differences in the safety profiles of the two arms, and (though lacking formal analysis), “there was a strong suggestion for improved quality of life at 6 months with the addition of SBRT,” according to Dr. Dawson.
Levels of Cell-Free DNA in Colorectal Cancer
A large-scale study using real-world data from patients with resected colorectal cancer provided reassurance that testing for measurable residual disease (MRD) status may be performed as early as 15 days after surgery.9 The study found that the levels of cell-free DNA in plasma do not appear to significantly affect ctDNA detection, contrary to what many clinicians have assumed, said Stacey A. Cohen, MD, of the Fred Hutchinson Cancer Center and University of Washington, Seattle.
“Circulating tumor DNA has emerged as a strong prognostic and predictive biomarker in colorectal cancer. Ultra-deep sequencing allows us to detect miniscule amounts of ctDNA in a background of nontumor-derived cell-free DNA. In certain clinical scenarios (such as after surgery and during systemic therapy), a surge in cell-free DNA could potentially decrease the assay’s sensitivity, so the ideal timing to test for ctDNA after such events has yet to be determined,” Dr. Cohen said. In addition, she noted, concerns about the interplay of these factors may lead clinicians to delay MRD assessment and therefore treatment.

Stacey A. Cohen, MD
Dr. Cohen continued: “The question is whether higher cell-free DNA concentrations affect ctDNA detection sensitivity or can MRD be checked earlier than 4 weeks with confidence?”
This retrospective study using data from 14,425 patients with stage I to III operable colorectal cancer was undertaken to understand how cell-free DNA levels and the timing of blood samples impact MRD monitoring. Patients underwent commercial ctDNA testing and had one surgery date reported. A subset of 450 patients had fully annotated clinical information. Levels of ctDNA prior to surgery and afterward were quantified, and the kinetics of total cell-free DNA were analyzed and compared with rates of ctDNA positivity at different postoperative time points.
Approximately 60% of first blood draws after surgery for ctDNA were within the MRD window of 0 to 12 weeks. Analysis showed that levels of cell-free DNA were noticeably higher in the immediate postoperative 2-week period as well as when patients were receiving adjuvant chemotherapy. In addition, cell-free DNA concentrations 2 to 4 weeks after surgery were slightly higher than those seen in the 4- to 8-week time frame.
In the first 2 weeks after surgery, when cell-free DNA concentrations were highest, ctDNA positivity was more variable, but was detected in approximately 18% of patients. After 2 weeks, ctDNA positivity became more consistent and more akin to those from later time points. Beyond 2 weeks, although cell-free DNA levels remained high during weeks 2 to 4, ctDNA was consistently detected and was similar to that during weeks 4 to 12.
“This suggests that ctDNA testing may be performed 2 weeks or more after surgery,” she said. “Testing for MRD between weeks 2 and 4 showed similar sensitivity as between weeks 4 to 8.”
Analysis of a fully annotated cohort of 450 patients showed that ctDNA positivity at 2 to 8 weeks after surgery and during surveillance was associated with significantly worse recurrence-free survival compared with ctDNA negativity (HR = 14.1; P < .0001). Using the MRD timepoint, approximately 6% of ctDNA-negative patients recurred during follow-up, compared with 53% of ctDNA-positive patients, some of whom went on to adjuvant therapy.
“In the multivariate analysis, we saw no impact for cell-free DNA concentration; MRD remained the strongest predictor of recurrence-free survival,” Dr. Cohen said.
DISCLOSURE: Dr. Pavlakis has served as a consultant or advisor to Amgen, AstraZeneca, BeiGene, Boehringer Ingelheim, Bristol Myers Squibb, Eisai, Merck KGaA, Merck Serono, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Takeda. Dr. Chawla has received reimbursement for travel from Imugene Ltd and has served as a consultant or advisor to NATCO Pharma and Viatris. Dr. Shitara reported financial relationships with Bristol Myers Squibb, Janssen, Takeda, AbbVie, Amgen, Astellas Pharma, Boehringer Ingelheim, Daiichi Sankyo, GlaxoSmithKline, Guardant Health, Lilly, MSD, Novartis, Ono Pharmaceutical, Pfizer, and Taiho Pharmaceutical. Dr. Dawson holds a patent or royalties from Raysearch (image registration software license). Dr. Cohen reported financial relationships with the Association of Community Cancer Centers, Bayer, Istari Oncology, Eisai, Kallyope, Pfizer, and Taiho Oncology and has served as an expert witness for Helsell Fetterman and Robins Kaplan.
REFERENCES
1. Pavlakis N, Shitara K, Sjoquist KM, et al: INTEGRATE IIa: A randomised, double-blind, phase III study of regorafenib versus placebo in refractory advanced gastro-oesophageal cancer: A study led by the Australasian Gastro-intestinal Trials Group (AGITG). 2023 ASCO GI Cancers Symposium. Abstract LBA294. Presented January 19, 2023.
2. Pavlakis N, Sjoquist KM, Martin AJ, et al: Regorafenib for the treatment of advanced gastric cancer (INTEGRATE): A multinational placebo-controlled phase II trial. J Clin Oncol 34:2728-2735, 2016.
3. Maglakelidze M, Ryspayeva DE, Andric Z, et al: HERIZON: A phase 2 study of HER-Vaxx (IMU-131), a HER2-targeting peptide vaccine, plus standard of care chemotherapy in patients with HER2-overexpressing metastatic or advanced gastric/GEJ adenocarcinoma: Overall survival analysis. 2023 ASCO GI Cancers Symposium. Abstract 289. Presented January 19, 2023.
4. Bang YJ, Van Cutsem E, Feyereislova A, et al: Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 376:687-697, 2010.
5. Shitara K, Muro K, Watanabe J, et al: Negative hyperselection of patients with RAS wild-type metastatic colorectal cancer for panitumumab: A biomarker study of the phase III PARADIGM trial. 2023 ASCO GI Cancers Symposium. Abstract 11. Presented January 21, 2023.
6. Yoshino T, Uetake H, Tsuchihara K, et al: Rationale for and design of the PARADIGM study: Randomized phase III study of mFOLFOX6 plus bevacizumab or panitumumab in chemotherapy-naive patients with RAS (KRAS/NRAS) wild-type, metastatic colorectal cancer. Clin Colorectal Cancer 16:158-163, 2017.
7. Dawson LA, Winter KA, Knox JJ, et al: NRG/RTOG 1112: Randomized phase III study of sorafenib vs. stereotactic body radiation therapy followed by sorafenib in hepatocellular carcinoma. 2023 ASCO GI Cancers Symposium. Abstract 489. Presented January 20, 2023.
8. Finn RS, Qin S, Ikeda M, et al: Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 382:1894-1905, 2020.
9. Cohen SA, Kasi PM, Aushev VN, et al: Kinetics of postoperative circulating cell-free DNA and impact on minimal residual disease detection rates in patients with resected stage I-III colorectal cancer. 2023 ASCO GI Cancers Symposium. Abstract 5. Presented January 21, 2023.

