In the treatment of resectable, locally advanced rectal cancer, researchers are trying to identify the most effective chemotherapy regimens, the best radiotherapy approaches, and the optimal sequence of these modalities. Two phase III trials presented during the ASCO20 Virtual Scientific Program showed that neoadjuvant chemotherapy is effective in this setting.1,2
RAPIDO was an international, phase III randomized trial of total neoadjuvant therapy involving a short course of radiation followed by six courses of CAPOX (capecitabine and oxaliplatin) and then surgery. This approach improved outcomes over capecitabine-based chemoradiotherapy, followed by surgery and optional adjuvant CAPOX.1
In PRODIGE 23, multiple endpoints favored six cycles of neoadjuvant modified FOLFIRINOX (leucovorin, infusional fluorouracil [5-FU; no bolus], irinotecan, oxaliplatin) given before chemoradiation, followed by surgery and 3 months of adjuvant chemotherapy, as compared with preoperative chemoradiotherapy with capecitabine, followed by surgery, and then 6 months of adjuvant chemotherapy.2
RAPIDO Rationale and Design
The principal investigator of RAPIDO, Geke A.P. Hospers, MD, Professor at the University Medical Center Groningen in the Netherlands, noted that improvements in preoperative treatment and surgical resection by anatomic boundaries, or total mesorectal excision, have greatly decreased the risk of local recurrence, but the main problem is now distant metastasis. “The aim of the RAPIDO trial is to reduce distant metastases without an increase in local failure,” she said.

Geke A.P. Hospers, MD
A short course of radiotherapy, followed by delayed surgery—with chemotherapy given in the interval—may improve compliance, downstage the tumor, and result in fewer distant metastases, according to Dr. Hospers.
In the RAPIDO trial, patients had locally advanced rectal cancer with cT4a/b, extramural vascular invasion, cN2, involved mesorectal fascia, or enlarged lateral lymph nodes considered to be metastatic. They were randomly assigned to one of the following regimens:
Experimental arm: Short-course radiotherapy (5 × 5 Gy) with subsequent six cycles of CAPOX or nine cycles of FOLFOX4 (leucovorin, 5-FU, oxaliplatin), followed by total mesorectal excision after 23 to 24 weeks.
Control arm: Capecitabine-based chemoradiotherapy (25–28 × 2.0–1.8 Gy) followed by total mesorectal excision and optional postoperative treatment with 8 cycles of CAPOX or 12 cycles of FOLFOX4.
Since some patients “never become disease-free,” Dr. Hospers said, the study’s primary endpoint was disease-related treatment failure at 3 years, notably including distant metastasis, locoregional failure, new primary colorectal cancer, and treatment-related death.
The study enrolled 920 patients (median age, 62 years). Baseline characteristics and high-risk features were well balanced between the arms. With standard treatment, 89% of the patients underwent rectal surgery with curative intent within 6 months, as did 92% of the experimental arm. In the standard-treatment arm, 187 patients (41.4%) received adjuvant chemotherapy. Ultimately, 460 in the experimental and 441 in the control arms started treatment, with surgical and pathology analyses available for 821 patients.
There were more grade ≥ 3 toxicities during preoperative treatment in the experimental arm, including more neurologic toxicity (4.3% vs 0.2%), more vascular disorders (8.5% vs 4.1%), and more diarrhea (17.6% vs 9.3%). During postoperative adjuvant therapy, diarrhea was observed in 7% of the control arm.
After neoadjuvant treatment, an R0 resection rate of around 90% was achieved in both treatment arms with no differences in different surgical approaches and surgical complication rates. However, the experimental arm achieved more pathologic complete responses (28% vs 14%; P < .001), Dr. Hospers reported.
The primary endpoint was met, with the experimental arm experiencing fewer treatment failures at 3 years: 23.7% vs 30.4% (hazard ratio [HR] = 0.75; P = .019). The difference was mainly due to a significant decrease in distant metastases, which occurred in 20.0% vs 26.8%, respectively (HR = 0.69; P = .005). Locoregional failure rates trended in reverse, with rates of 8.7% vs 6.0%, respectively (HR = 1.45; P = .09).
After a median follow-up of approximately 4.5 years, 3-year overall survival was comparable: 89.1% in the experimental arm and 88.8% in the control arm (HR = 0.92; P = .59). No significant differences were seen in overall health, overall quality of life, or scores for low anterior resection syndrome.
Dr. Hospers summarized the following findings showing advantages for short-course radiotherapy followed by CAPOX and then total mesorectal excision:
- 7% lower disease-related treatment failure: 30.4% vs 23.9%
- 7% lower distant metastases rate: 26.8% vs 20.0%
- Doubling in pathologic complete response rate: 14% vs 28%
- Similar 3-year overall survival: 89% in each
- No unexpected toxicity
- No differences in surgical or postoperative complications or quality of life.
PRODIGE 23 Aims to Validate Neoadjuvant Chemotherapy
The phase III PRODIGE 23 trial, sponsored by the French UNICANCER GI group, investigated the role of neoadjuvant modified FOLFIRINOX before standard chemoradiotherapy, followed by surgery and the same adjuvant chemotherapy in

Thierry Conroy, MD
both arms. The study aimed to extend the promising results previously shown for total neoadjuvant therapy and perhaps show evidence of improved survival, “which has been lacking from a randomized trial,” Thierry Conroy, MD, Director of the Institut de Cancerologie de Lorraine in Nancy and Professor at the Lorraine University in France, said.
The final analysis presented at ASCO20 found that neoadjuvant modified FOLFIRINOX plus chemoradiotherapy significantly increased the probability of pathologic complete response, curative-intent surgery, disease-free survival, and metastasis-free survival over standard chemoradiotherapy followed by surgery, and then adjuvant chemotherapy.
“Total neoadjuvant therapy with modified FOLFIRINOX should now be considered a new option of care for the initial management of patients with T3 or T4 rectal cancer,” Professor Conroy stated.
PRODIGE 23 Details
The study randomly assigned 461 patients from 35 French centers to the following treatments:
Chemoradiation (control arm): Preoperative chemoradiotherapy (50.4 Gy over 5 weeks) plus capecitabine (1,600 mg/m2 for 5 days of 7), followed 7 weeks later by total mesorectal excision and then 6 months of modified FOLFOX or six or eight cycles of capecitabine as adjuvant chemotherapy
Total neoadjuvant therapy: Six cycles of modified FOLFIRINOX followed by the same preoperative long-term chemoradiotherapy as the control arm, followed 7 weeks later by total mesorectal excision and then six cycles of modified FOLFOX6 or four cycles of capecitabine as adjuvant chemotherapy.
“In both groups, patients received the same schedule of chemoradiation, the same surgery, and the same total duration of chemotherapy—6 months,” Professor Conroy noted.
The study’s primary endpoint was disease-free survival. Median follow-up was 46.5 months. More than 90% of patients received all planned cycles of FOLFIRINOX. Total neoadjuvant therapy did not reduce patient compliance with radiotherapy, and only 8% of this arm discontinued capecitabine before completing radiation.
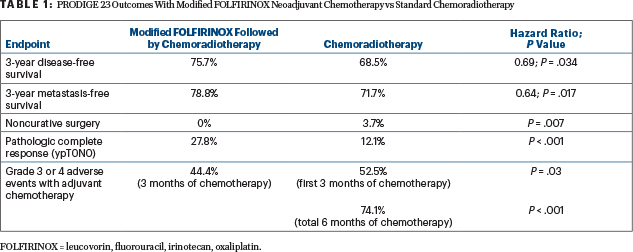
FOLFIRINOX was reported to be well tolerated; grade 3 or 4 febrile neutropenia occurred in 17% and grade 3 or 4 diarrhea, in 11%. One patient experienced sudden death. Surgical morbidity was similar. There were significantly more grade 3 or 4 adverse events in the chemoradiation group, especially neutropenia and neuropathy. “This confirmed that, for the same duration of chemotherapy, the perioperative approach is better tolerated than adjuvant chemotherapy,” commented Prof. Conroy.
Multiple Endpoints Favored Total Neoadjuvant Therapy
Total neoadjuvant therapy proved significantly more beneficial for multiple outcomes, including the primary endpoint (Table 1). Global quality-of-life scores were similar, but less impotence (P = .077) and a longer time to deterioration (P = .006) were reported with total neoadjuvant therapy.
DISCLOSURE: Dr. Hospers has served as an institutional consultant or advisor to Amgen, Bristol-Myers Squibb, MSD, Novartis, and Roche and has received institutional research funding from Bristol Myers Squibb and Seerave Foundation. Dr. Conroy reported no conflicts of interest.
REFERENCES
1. Hospers G, Bahadoer RR, Dijkstra EA, et al: Short-course radiotherapy followed by chemotherapy before total mesorectal excision in locally advanced rectal cancer: The randomized RAPIDO trial. ASCO20 Virtual Scientific Program. Abstract 4006.
2. Conroy T, Lamfichekh N, Etienne PL, et al: Total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiation in patients with locally advanced rectal cancer: Final results of PRODIGE 23 phase III trial, a UNICANCER GI trial. ASCO20 Virtual Scientific Program. Abstract 4007.


