“Nothing in life is to be feared, it is only to be understood.
Now is the time to understand more, so that we may fear less.”
—Marie Curie
The ASCO Post is pleased to present Hematology Expert Review, an ongoing feature that quizzes readers on issues in hematology. In this installment, Drs. Abutalib, Hashmi, and Porter explore the chimeric antigen receptor (CAR) T-cell gene therapy, focusing on managing the well-recognized adverse events specific to the U.S. Food and Drug Administration (FDA)-approved anti-CD19 CAR T-cell therapies (Table 1). For each quiz question that follows, select the one best answer. The correct answers and accompanying discussions appear below.
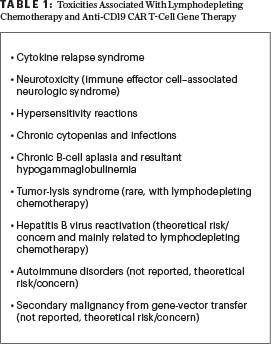
Recently, the FDA and European Medical Association approved two anti-CD19 CAR T-cell therapy products: tisagenlecleucel and axicabtagene ciloleucel. Tisagenlecleucel is approved for patients up to 25 years of age with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL) and for adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy including diffuse large B-cell lymphoma (DLBCL) not otherwise specified, high grade B-cell lymphoma, and DLBCL arising from follicular lymphoma. Axicabtagene ciloleucel is also approved for relapsed or refractory primary mediastinal large B-cell lymphoma, in addition to lymphoma subtypes approved for tisagenlecleucel; however, unlike tisagenlecleucel, it is not approved for patients with B-cell ALL.
Both CAR T-cell products can result in severe and potentially life-threatening side effects and complications; the most unique adverse events are cytokine-release syndrome and neurologic toxicities. Both cytokine-release syndrome and neurologic toxicities must be managed by experienced, specially qualified practitioners and require specialized care and allogeneic Foundation for the Accreditation of Cellular Therapy–approved centers; strategies to improve the safety of CAR T-cell therapies without a negative impact on efficacy are of prime importance.1
GUEST EDITORS
Dr. Abutalib is Associate Director, Hematology and Cellular Therapy Program; Director, Clinical Apheresis Program, Cancer Treatment Centers of America, Zion, Illinois; Associate Professor, Roseland Franklin University of Medicine and Science; Founder and Co-Editor, Advances in Cell and Gene Therapy. Dr. Hashmi is Clinical Fellow, Division of Blood and Marrow Transplant and Cellular Immunotherapy, Moffitt Cancer and Research Center, Tampa, Florida. Dr. Porter is Director, Cell Therapy and Transplantation; Jodi Fisher Horowitz Professor in Leukemia Care Excellence, Abramson Cancer Center and Perelman School of Medicine, University of Pennsylvania, Philadelphia.Of note, there is significant heterogeneity in the side-effect profile of different CAR T-cell products. Not all CAR T-cell therapy products are created equal (tisagenlecleucel ≠ axicabtagene ciloleucel)! However, interpretation of toxicities across studies needs to be done with caution because different diseases, disease stages, trial designs, populations (adult and/or pediatric), and toxicity grading scales are uniquely represented in each study.1-3
Question 1
Which of the following statements about cytokine-release syndrome and neurotoxicity after tisagenlecleucel infusion is correct?

Syed Ali Abutalib, MD

Hamza Hashmi, MD

David L. Porter, MD
A. Cytokine-release syndrome was observed in 40% to 50% of pediatric and young adult patients with relapsed or refractory ALL and adult patients with relapsed or refractory large B-cell lymphomas.
B. The median time to onset of cytokine-release syndrome was 3 days.
C. Severe neurologic toxicities were observed in 30% to 40% of pediatric and young adult patients with relapsed/refractory B-cell ALL and adult patients with relapsed/refractory large B-cell lymphomas.
D. The median time to onset of neurotoxicity was 10 days.
Question 2
Which of the following statements about cytokine-release syndrome and neurotoxicity after axicabtagene ciloleucel infusion is correct?
A. The median time to onset of cytokine-release syndrome was 5 days.
B. Neurotoxicity was observed in 50% of adult patients with large B-cell lymphomas.
C. Most neurotoxicity cases occurred after 8 weeks of infusion.
D. The median time to onset of neurotoxicity was 4 days.
Question 3
Approximately what proportion of patients develop infection(s) after the first month of CAR T-cell therapy platform (lymphodepleting therapy plus anti-CD 19 CAR T-cell infusion) despite being on appropriate antimicrobial prophylaxis?
A. 10% to 15%
B. 20% to 40%
C. 50% to 60%
D. 70% to 80%
Question 4
Which statement about the diagnosis and management of cytokine-release syndrome after CAR T-cell infusion is correct?
A. Tocilizumab is given to all patients with cytokine-release syndrome.
B. Prophylactic steroids are recommended to prevent cytokine-release syndrome.
C. Careful and timely exclusion of an alternative diagnosis is important.
D. All of the above
Question 5
Which of the following statements about anti-CD19 CAR T-cell–associated chronic hypogammaglobulinemia is correct?
A. Hypogammaglobulinemia was reported in 43% of patients treated with tisagenlecleucel for relapsed or refractory ALL.
B. Hypogammaglobulinemia was reported in 14% of patients treated with tisagenlecleucel for relapsed or refractory large B-cell lymphoma.
C. Hypogammaglobulinemia was reported in 15% of patients treated with axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma.
D. All of the above
Question 6
Which of the following statements about the diagnosis and management of neurotoxicity after CAR T-cell infusion is correct?
A. The majority of patients present with partial seizures.
B. Steroids are given to all patients with neurotoxicity.
C. Neurotoxicity may occur without preceding cytokine-release syndrome.
D. Tocilizumab is given to all patients with neurotoxicity.

