As first-line therapy for advanced esophageal cancer, pembrolizumab added to chemotherapy improved overall survival in the KEYNOTE-590 population. Not only did patients with high PD-L1 expression benefit, the value of checkpoint inhibition was observed for the whole population, the trial’s investigators reported at the European Society for Medical Oncology (ESMO) Virtual Congress 2020.1
“Unfortunately, for esophageal cancer, the standard of care has remained relatively unchanged for a long time…. Pembrolizumab monotherapy has shown antitumor activity with an acceptable safety profile in advanced and metastatic esophageal cancers, based on two key studies,” said coauthor Peter Enzinger, MD, speaking at a press conference. Dr. Enzinger is Director of the Center for Esophageal and Gastric Cancer at Dana-Farber Cancer Institute, Boston.

“Pembrolizumab plus chemotherapy should be a new standard of care as first-line therapy for patients with locally advanced/unresectable or metastatic esophageal cancer….”— Peter Enzinger, MD
Tweet this quote
These studies—KEYNOTE-1802 and KEYNOTE-1813—led to the drug’s approval by the U.S. Food and Drug Administration for patients with esophageal squamous cell carcinoma and a PD-L1 Combined Positive Score (CPS) ≥ 10 who have experienced disease progression after at least one prior line of therapy.
The global phase III KEYNOTE-590 trial took pembrolizumab into the first-line setting, and with the success shown in those patients, the treatment algorithm has been further remodeled. “Pembrolizumab plus chemotherapy should be a new standard of care as first-line therapy for patients with locally advanced/unresectable or metastatic esophageal cancer, including the gastroesophageal junction, regardless of histology and biomarker status,” Dr. Enzinger said.
KEYNOTE-590
KEYNOTE-590 ENROLLED 749 patients, regardless of PD-L1 expression, with previously untreated advanced/unresectable or metastatic esophageal adenocarcinoma (26%) or esophageal squamous cell carcinoma or gastroesophageal junction Siewert type 1 adenocarcinoma (74%). Half of all patients had tumors with a PD-L1 CPS ≥ 10, and half the population was Asian.

Ken Kato, MD
Patients were randomly assigned to pembrolizumab at 200 mg every 3 weeks for up to 35 cycles plus chemotherapy (fluorouracil plus cisplatin) for up to six cycles or chemotherapy alone. The dual primary endpoints of the study were overall survival and progression-free survival; these outcomes were evaluated in the overall population, in patients with a PD-L1 CPS ≥10, and according to cancer type.
Interim Analysis
In the interim analysis reported at an ESMO Presidential Symposium by Ken Kato, MD, of the National Cancer Center Hospital in Tokyo, median overall survival in all patients was 12.4 months with pembrolizumab plus chemotherapy vs 9.8 months with chemotherapy (hazard ratio [HR] = 0.73, P < .0001; see Table 1 on page 67). “All endpoints that were assessed were met,” Dr. Kato announced.
Survival rates were 51% with pembrolizumab plus chemotherapy and 39% with chemotherapy at 1 year and 28% and 16%, respectively, at 2 years. Median progression-free survival was 6.3 vs 5.8 months, respectively (HR = 0.65; P < .0001), and the objective response rate was 45.0% with pembrolizumab and 29.3% without (P < .0001).
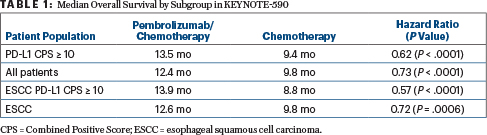
Among patients with a PD-L1 CPS ≥ 10, median overall survival was 13.5 months with pembrolizumab plus chemotherapy, compared with 9.4 months with chemotherapy alone (HR = 0.62; P < .0001), and median progression-free survival was 7.5 months and 5.5 months, respectively (HR = 0.51; P < .0001). Similar progression-free survival benefits were shown for the squamous cell cohort (HR = 0.65; P < .0001) and all patients (HR = 0.65; P < .0001).
Adverse events were similar between the arms. Grade ≥ 3 treatment-related adverse events were seen in 71.9% on the pembrolizumab arm and 67.6% on the control arm. Those events leading to treatment discontinuation were seen in 19.5% and 11.6%, respectively, and those events resulting in death occurred in 2.4% and 1.4%.
DISCLOSURE: KEYNOTE-590 was sponsored by Merck Sharp & Dohme Corp. Dr. Enzinger has served as a consultant or advisor to Astellas Pharma, AstraZeneca, Celgene, Daiichi Sankyo, Loxo, Merck, Taiho Pharmaceutical, Takeda, and Zymeworks. Dr. Kato has received honoraria from Bristol Myers Squibb, Eli Lilly, and Ono Pharmaceutical; has served as a consultant or advisor to BeiGene, MSD, Oncolys BioPharma, and Ono Pharmaceutical; and has received institutional research funding from BeiGene, MSD Oncology, Ono Pharmaceutical, and Shionogi.
REFERENCES
1. Kato K, Sun J, Shah MA, et al: Pembrolizumab plus chemotherapy vs chemotherapy as first-line therapy in patients with advanced esophageal cancer: The phase III KEYNOTE-590 study. ESMO Virtual Congress 2020. Abstract LBA8_PR. Presented September 21, 2020.
2. Shah MA, Kojima T, Hochhauser D, et al: Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: The phase II KEYNOTE-180 study. JAMA Oncol 5:546-550, 2019.


