Longer follow-up data from the phase III CheckMate 649 trial support the use of nivolumab plus chemotherapy as a new standard first-line regimen in patients with advanced gastric cancer, gastroesophageal junction cancer, and esophageal adenocarcinoma. The findings were reported by Yelena Janjigian, MD, of Memorial Sloan Kettering Cancer Center, New York, at a Presidential Symposium of the European Society for Medical Oncology (ESMO) Congress 2021.1

Yelena Janjigian, MD
Although this regimen demonstrated a sustained benefit in patients with a PD-L1 combined positive score (CPS) ≥ 5 (hazard ratio [HR] = 0.70; 95% confidence interval [CI] = 0.61–0.81) and in all randomly assigned patients (HR = 0.79; 95% CI = 0.71–0.88), a third arm of the study—nivolu-mab plus ipilimumab—failed to improve outcomes over chemotherapy in the PD-L1 CPS ≥ 5 population (HR = 0.89; 96.5% CI = 0.71–1.10), based on new data.
“Nivolumab plus chemotherapy continues to demonstrate a clinically meaningful benefit in our first-line–treated patients. We showed sustained overall survival, progression-free survival, and an objective response rate improvement compared with chemotherapy. These data have already changed practice, and now it’s nice to have an additional 12 months of follow-up,” Dr. Janjigian commented. The combination is the first treatment in years to improve survival of patients with treatment-naive advanced or metastatic gastric cancer and is the first approved immunotherapy in this patient population.
On the other hand, she further reported, the same cannot be said about the combination of nivolumab and ipilimumab. “Nivolumab and ipilimumab did not significantly improve overall survival in patients with a PD-L1 CPS ≥ 5,” stated Dr. Janjigian. Since the hierarchically tested secondary endpoint of overall survival in patients with a PD-L1 CPS ≥ 5 was not met, overall survival in all randomly assigned patients was not statistically tested for this arm. The immunotherapy doublet also did not improve progression-free survival.
The results for nivolumab/chemotherapy were an update with longer follow-up. However, this was the first reporting of the results for nivolumab plus ipilimumab, and they were discouraging. Previous studies in heavily pretreated patients had appeared to be promising.
CheckMate 649
CheckMate 649 evaluated nivolumab plus chemotherapy vs chemotherapy alone as well as nivolumab plus ipilimumab vs chemotherapy alone as first-line treatment in patients with non-HER2–positive advanced gastric cancer, -gastroesophageal junction cancer, or esophageal -adenocarcinoma. The 1,581 patients from sites in 29 countries were randomly assigned to the following regimens:
Nivolumab at 360 mg plus XELOX (oxaliplatin, capecitabine) or nivolumab at 240 mg plus FOLFOX (fluorouracil, leucovorin, oxaliplatin)
XELOX or FOLFOX
Nivolumab at 1 mg/kg plus ipilimumab at 3 mg/kg for four cycles then nivolumab at 240 mg every 2 weeks (stopped early in 2018 based on data monitoring committee recommendation).
There were dual primary endpoints: overall survival and progression-free survival (by blinded independent central review) for nivolumab plus chemotherapy vs chemotherapy in patients with a PD-L1 CPS ≥ 5. Under hierarchical testing, the secondary endpoints included overall survival for nivolumab plus chemotherapy, vs chemotherapy, in those with a PD-L1 CPS ≥ 1 and all randomly assigned patients; and overall survival for nivolumab plus ipilimumab vs chemotherapy in those with a PD-L1 CPS ≥ 5 and all randomly assigned patients.
KEY POINTS
- In the long-term follow-up of CheckMate 649, the addition of nivolumab to chemotherapy continued to produce a significant improvement in overall survival, with the greatest magnitude seen in patients with high PD-L1–expressing tumors.
- Nivolumab plus ipilimumab, on the other hand, failed to show a significant benefit.
“All primary and hierarchically tested secondary endpoints for nivolumab plus chemotherapy were met at the 12-month follow-up,” Dr. Janjigian announced.
The minimum follow-up was 24.0 months in the nivolumab/chemotherapy arm and 35.7 months in the nivolumab/ipilimumab arm. The median duration of treatment was 6.8 months for nivolumab/chemotherapy, 1.9 months for nivolumab/ipilimumab, and 4.9 months for chemotherapy alone. More patients discontinued the study due to adverse events in the nivolumab/ipilimumab arm, 19% vs 8% for those given nivolumab/chemotherapy and 6% for those given chemotherapy alone.
Overall Survival Benefit Maintained With Nivolumab/Chemotherapy
The previous report was based on a median follow-up of 13.1 months in the nivolumab group and 11.1 months in the chemotherapy group.2 Then, in the PD-L1 CPS ≥ 5 population, median overall survival was 14.4 months with nivolumab/chemotherapy vs 11.1 months with chemotherapy (HR = 0.71; P < .0001). Among all randomly assigned patients, it was 13.8 months vs 11.6 months, respectively (HR = 0.80; P = .0002).
“With longer follow-up, a clinically meaningful improvement in overall survival with nivolumab plus chemotherapy was maintained,” Dr. Janjigian reported (see Table 1). Hazard ratios were “directionally improved” relative to those previously observed, she added, and the progression-free survival benefit was maintained.
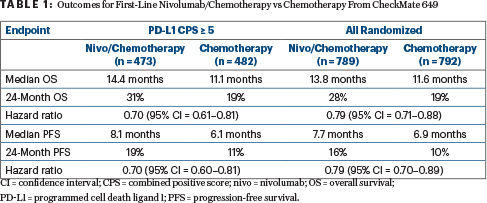
“Overall response rates remained higher, and responses were more durable, with nivolumab/chemotherapy vs chemotherapy, with longer follow-up, and responses deepened, relative to the 12-month follow-up,” she said. An additional 11 patients (6 among all randomized patients, 5 with CPS ≥ 5) achieved complete responses in the experimental arm vs none in the control arm.
Objective response rates were 60% with nivolumab/chemotherapy vs 45% with chemotherapy for the population with PD-L1 CPS ≥ 5and 58% with nivolumab/chemotherapy vs 46% with chemotherapy for all randomized patients.
Nivolumab Plus Ipilimumab: A Different Story
In contrast to the findings for nivolumab/chemotherapy, the combination of nivolumab and ipilimumab produced no significant benefit: “After long-term follow-up, there is a crossover at 12 months; thereafter, the overall survival curves remained separated in favor of nivolumab/ipilimumab, although this difference was not meaningful or statistically significant,” Dr. Janjigian noted.
In the PD-L1 CPS ≥ 5 population, median overall survival was 11.2 months with nivolumab/ipilimumab vs 11.6 months with chemotherapy (HR = 0.89; P = .2302). At 24 months, 25% and 17% of patients were alive, respectively. Median progression-free survival was 2.8 months vs 6.3 months, respectively (HR = 1.42).
In all randomly assigned patients, median overall survival was 11.7 and 11.8 months (HR = 0.91; P value not tested). Median progression-free survival was 2.8 vs 7.1 months, respectively (HR = 1.66).
Efficacy by MSI Status and Subsequent Therapy
The group of 44 patients with high microsatellite instability (MSI-H) derived a particular benefit from nivolumab/chemotherapy, with responses seen in 55% vs 39% with chemotherapy, and a median overall survival of 38.7 months vs 12.3 months (HR = 0.38; 95% CI = 0.17–0.84). For the microsatellite-stable population, benefit was aligned with that of the whole population (HR = 0.78; 95% CI = 0.70–0.88).
Furthermore, patients with MSI-H tumors had longer overall survival and higher response rates when treated with nivolumab/ipilimumab, but this sample included just 21 patients. The immunotherapy doublet led to a median overall survival that was not reached, compared with 10.0 months with chemotherapy (HR = 0.28; 95% CI = 0.08–0.92). No benefit was seen with the immunotherapy doublet in microsatellite-stable patients.
“Nivolumab and ipilimumab did not significantly improve overall survival in patients with a PD-L1 CPS ≥ 5.”— —YELENA JANJIGIAN, MD
Tweet this quote
Subsequent treatment was delivered to 42% of patients randomly assigned to nivolumab/chemotherapy and chemotherapy alone and 47% of those given nivolumab/ipilimumab and chemotherapy alone. The predominant therapy across all arms was chemotherapy. Subsequent immunotherapy was received by 2% to 3% of patients in the nivolumab-containing arms and 9% to 12% of those in the chemotherapy arms.
Treatment-Related Adverse Events
The most common grade 3 or 4 treatment-related adverse events for the nivolumab/chemotherapy arm were neutropenia (15%), decreased neutrophil count (11%), and anemia (6%); for nivolumab/ipilimumab, they were increased lipase (7%), increased amylase (4%), increased alanine aminotransferase (4%), and increased aspartate aminotransferase (4%). Patients given chemotherapy were most likely to have neutropenia (11%–13%), decreased neutrophil count (9%–10%), and diarrhea (3%–4%).
Treatment-related adverse events leading to treatment discontinuation were highest for the nivolumab/chemotherapy arm, 38% (18% grade 3 or 4). There were 16 (2%) treatment-related deaths in that arm and 10 (2%) with nivolumab/ipilimumab, compared with < 1% for the chemotherapy arms.
Treatment-related adverse events potentially of immunologic etiology were mainly grade 1 or 2. Grade 3 or 4 events occurred in up to 5% of patients given nivolumab/chemotherapy and up to 12% of those given the two checkpoint inhibitors, across organ categories.
“It’s important to note that grade 3 or 4 treatment-related adverse events were highest (23%) in the nivolumab/ipilimumab arm, which is expected with a higher dose of ipilimumab,” stated Dr. Janjigian. “The side-effect profile with nivolumab-containing arms depends on what you’re going to combine it with. With the nivolumab/chemotherapy combination, there are both side effects from chemotherapy and immune-related side effects, and clearly with nivolumab/ipilimumab, these adverse events are mostly immune-related side effects.”
DISCLOSURE: Dr. Janjigian has received compensation from AstraZeneca, Bayer, Basilea Pharmaceutica, Bristol Myers Squibb, Daiichi Sankyo, Eli Lilly, Imugene, Merck, Merck Serono, Michael J. Hennessy Associates, Paradigm Medical Communications, Pfizer, Rgenix, Seattle Genetics, and Zymeworks; has received research funding from Bayer, Bristol Myers Squibb, Cycle for Survival, Department of Defense, Eli Lilly, Fred’s Team, Genentech/Roche, Merck, NCI, and Rgenix; and holds stock in Rgenix.
REFERENCES
1. Janjigian YY, Ajani JA, Moehler M, et al: Nivolumab plus chemotherapy or ipilimumab vs chemotherapy as first-line treatment for advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma: CheckMate 649 study. ESMO Congress 2021. Abstract LBA7. Presented September 19, 2021.
2. Janjigian YY, Shitara K, Moehler M, et al: First-line nivolumab plus chemotherapy vs chemotherapy alone for advanced gastric, gastroesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase III trial. Lancet 398:27-40, 2021.


