In women with advanced ovarian cancer responding to first-line chemotherapy, maintenance therapy with the poly (ADP-ribose) polymerase (PARP) inhibitor niraparib significantly reduced the risk of disease progression by 38% overall and by 60% in women with BRCA mutations. Even patients without a homologous recombination deficiency (HRD) derived advantage from this treatment, in the phase III PRIMA/ENGOT-OV26/GOG-3012 trial presented at the European Society for Medical Oncology (ESMO) Congress 2019 1 and concurrently reported in The New England Journal of Medicine.2

Antonio González-Martín, MD, PhD
“Niraparib is the first PARP inhibitor to demonstrate benefit in patients across biomarker subgroups after front-line platinum-based chemotherapy, consistent with prior clinical studies of niraparib in recurrent ovarian cancer,” said Antonio González-Martín, MD, PhD, of Español de Investigación en Cáncer de Ovario and Clinica Universidad de Navarra, Spain.
Details of PRIMA Trial
This double-blind, placebo-controlled phase III study evaluated maintenance therapy with niraparib vs placebo in 733 women with advanced ovarian cancer after response to platinum-based chemotherapy.1 All patients had newly diagnosed advanced high-grade serous or endometrioid ovarian, primary peritoneal, or fallopian tube cancer.
Patients could have any BRCA mutation status and any HRD status, according to the Myriad Genetics myChoice HRD assay (HRD score ≥ 42 for positive). All patients had achieved a complete or partial response to first-line platinum-based chemotherapy. Although patients could receive bevacizumab with first-line chemotherapy, maintenance bevacizumab was not allowed. Neoadjuvant chemotherapy was permitted.
It is important to note that unlike the patient population included in the other two phase III PARP inhibitor trials reported during ESMO’s Presidential Session,3,4 patients with stage III disease in the PRIMA trial were required to have visible residual disease after debulking and thus were considered a more high-risk population. Other patients in the PRIMA trial had inoperable stage III disease or any stage IV disease.
Patients were randomly assigned 2:1 to receive oral niraparib or placebo once daily in 28-day cycles for 36 months. Initially, all patients started at 300 mg/d, but this schedule was amended to incorporate an individualized dose of 200 mg/d in patients with a lower baseline body weight or platelet count. Patients were assessed every 12 weeks.
The primary endpoint was progression-free survival by blinded independent central review in the whole population and in HRD-positive patients, as determined on hierarchic testing. Overall survival was a key secondary endpoint.
Risk of Disease Progression Reduced by 38%
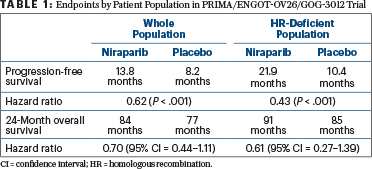
Patients receiving maintenance niraparib experienced significant benefits in terms of progression-free (hazard ratio [HR] = 0.62) and overall survival (HR = 0.70; Table 1). In the 51% of the population with HRD-positive tumors, these benefits were especially notable for both progression-free (HR = 0.43) and overall survival (HR = 0.61).
The progression-free survival benefits were observed in all HRD subsets, as shown below for the niraparib and placebo arms, respectively:
- HRD/BRCA-mutated population: 22.1 vs 10.9 months (HR = 0.40; 95% confidence interval [CI] = 0.27–0.62)
- HRD/BRCA-wild type population: 19.6 vs 8.2 months (HR = 0.50; 95% CI = 0.31–0.83)
- HRD-proficient population: 8.1 vs 5.4 months (HR = 0.68; 95% CI = 0.49–0.94).
In the preliminary analysis, a benefit in overall survival was observed with niraparib. However, in the published study, the investigators cautioned that the data are not sufficiently mature to assess this endpoint with precision.
Safety Profile
The most commonly reported grade ≥ 3 adverse events with niraparib were anemia in 31% of patients, thrombocytopenia in 29%, and neutropenia in 13%. Dose reductions were made in 71% of patients treated with niraparib; 12% of the niraparib group vs 2.5% of the placebo group discontinued the study because of adverse events. No treatment-related deaths were reported, and no differences were observed between the arms in terms of health-related quality of life.
DISCLOSURE: The PRIMA/ENGOT-OV26/GOG-3012 was funded by Tesaro. Dr. González-Martín has served as a consultant or advisor for AstraZeneca, Tesaro, and PharmaMar.
REFERENCES
1. González-Martín A, Pothuri B, Vergote I, et al: Niraparib therapy in patients with newly diagnosed advanced ovarian cancer. ESMO Congress 2019. Abstract LBA1. Presented September 28, 2019.
2. González-Martín A, Pothuri B, Vergote I, et al: Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 381:2391-2402, 2019.
3. Ray-Coquard IL, Pautier P, Pignata S, et al: Phase III PAOLA-1/ENGOT-ov25 trial. ESMO Congress 2019. Abstract LBA2_PR. Presented September 28, 2019.
4. Coleman RL, Fleming GF, et al: Integration of veliparib with front-line chemotherapy and maintenance in high-grade serous carcinoma of ovarian, fallopian tube, or primary peritoneal origin. ESMO Congress 2019. Abstract LBA3. Presented September 28, 2019.


