
In this installment of The ASCO Post’s Hematology Expert Review, we take a closer look at the monoclonal antibody targeting CD38, daratumumab, in the treatment of amyloid light chain (AL) amyloidosis and resistant multiple myeloma as well as the antibody-drug conjugate belantamab mafodotin-blmf, which was granted accelerated approval in August in the treatment of patients with relapsed or resistant multiple myeloma.
Newly Diagnosis of AL Amyloidosis
Clinical Trial: Subcutaneous daratumumab plus cyclophosphamide, bortezomib, and dexamethasone for patients with newly diagnosed AL amyloidosis (stages I, II and IIIa; n = 28): Safety run-in results of ANDROMEDA (ClinicalTrials.gov identifier NCT03201965)1,2
Background: The regimen of cyclophosphamide, bortezomib, and dexamethasone is considered standard of care for patients with AL amyloidosis despite not being approved by the U.S. Food and Drug Administration (FDA) for this condition. Palladini et al reported their experience with AL amyloidosis treated with front-line cyclophosphamide, bortezomib, and dexamethasone.3 In this large series of 230 patients, the overall hematologic response rate was 60%, including complete remission in 23% of patients. After a median follow-up of 25 months, cardiac and renal responses were obtained in 17% and 25%, respectively. Regarding patients with advanced cardiac disease, those with N-terminal pro b-type natriuretic peptide (NT-proBNP) levels higher than 8,500 ng/L (stage IIIb) had lower hematologic response rates (42%, complete remission of 14%) and poorer survival (median of 7 months) due to the inability of cyclophosphamide, bortezomib, and dexamethasone to reduce the early mortality in this high-risk population.
Based on outcomes with daratumumab in multiple myeloma, the phase III ANDROMEDA study is evaluating the safety and efficacy of daratumumab, cyclophosphamide, bortezomib, and dexamethasone vs cyclophosphamide, bortezomib, and dexamethasone in newly diagnosed AL amyloidosis.
Methods: Participants had previously untreated AL amyloidosis with at least one involved organ, an Eastern Cooperative Oncology Group score of up to 2, an estimated glomerular filtration rate ≥ 20 mL/min/1.73m2, and an NT-ProBNP level ≤ 8,500 ng/L (excluding patients with stage IIIb). Patients had a median of two involved organs (renal, 68%; cardiac, 61%) and received a median of 16 (range, 1–23) treatment cycles.
Results: Treatment-emergent adverse events were consistent with those associated with subcutaneous daratumumab in multiple myeloma and cyclophosphamide, bortezomib, and dexamethasone. No grade 5 treatment-emergent adverse events occurred; five patients died, including three after autologous transplantation. The overall hematologic response rate was 96%, with a complete hematologic response in 15 patients (54%). Renal response occurred in 10 of 15 patients at 12 months, and cardiac response occurred in 8 of 13 patients at 12 months. Hepatic response occurred in 2 of 3 patients at 12 months.
“A multidisciplinary approach involving close collaboration between ophthalmologists and hematologists/oncologists is central in treating patients with belantamab mafodotin.”— Syed Ali Abutalib, MD, and Luciano J. Costa, MD, PhD
Tweet this quote
Clinical Implications: The combination of daratumumab, cyclophosphamide, bortezomib, and dexamethasone was well tolerated and elicited superior hematologic and organ responses compared with the triplet combination in newly diagnosed AL amyloidosis.1,2 Most recently, the primary results of the ANDROMEDA study (n = 388) were presented as a late-breaking abstract in the virtual edition of the 2020 European Hematology Association Annual Congress.2 The primary endpoint of hematologic complete response was 53% with subcutaneous daratumumab, cyclophosphamide, bortezomib, dexamethasone compared with 18% with cyclophosphamide, bortezomib, dexamethasone (odds ratio = 5.1; 95% confidence interval [CI] = 3.2–8.2; P < .0001). Treatment with the quadruplet therapy also substantially delayed major organ deterioration, hematologic disease progression, or death and improved event-free survival.2 These findings favor the quadruplet therapy over the non-daratumumab–based triplet regimen as a new standard of care in patients with newly diagnosed AL amyloidosis.
Relapsed or Refractory Multiple Myeloma
Clinical Trial: Carfilzomib, dexamethasone, and daratumumab vs carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): Results from a randomized, multicenter, open-label, phase III study (NCT03158688)4
Background: In the phase III CASTOR study,5 daratumumab, bortezomib, and dexamethasone improved clinical outcomes compared with bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma. The randomized phase III ENDEAVOR study6 showed the superiority of carfilzomib over bortezomib.
The clinical benefit of combining carfilzomib with daratumumab was initially observed in the nonrandomized phase Ib MMY1001 study,7 which showed the tolerability and activity of daratumumab, carfilzomib, and dexamethasone in patients with multiple myeloma who were nearly entirely previously exposed to lenalidomide and 60% refractory to lenalidomide. At a time when many patients are experiencing disease progression on lenalidomide treatment, encouraging results from the MMY1001 study showed the daratumumab, carfilzomib, dexamethasone combination is a relevant and efficacious lenalidomide-free regimen and set a precedent for the randomized, multicenter phase III CANDOR study.
GUEST EDITORS
Dr. Abutalib is Associate Director, Hematology and Cellular Therapy Program; Director, Clinical Apheresis Program, Cancer Treatment Centers of America, Zion, Illinois; Associate Professor, Rosalind Franklin University of Medicine and Science; Founder and Co-Editor, Advances in Cell and Gene Therapy. Dr. Costa is Associate Professor and Chair, Hematologic Malignancies Working Group, O’Neal Comprehensive Cancer Center, The University of Alabama at Birmingham.
Methods: Participants were randomly assigned 2:1 to receive carfilzomib, dexamethasone, and daratumumab (n = 312) or carfilzomib and dexamethasone (n = 154). All patients received carfilzomib. Daratumumab (8 mg/kg) was administered intravenously on days 1 and 2 of cycle 1 and at 16 mg/kg weekly for the remaining doses of the first two cycles, then every 2 weeks for four cycles (cycles 3–6), and every 4 weeks thereafter. Patients received 40 mg of dexamethasone weekly (20 mg for patients ≥ 75 years starting on the second week). The primary endpoint was progression-free survival assessed by intention to treat. Adverse events were assessed in the safety population.
Results: Median progression-free survival was not reached with carfilzomib, dexamethasone, and daratumumab versus 15.8 months with carfilzomib and dexamethasone (hazard ratio = 0.63; 95% CI = 0.46–0.85; P = .0027), after a median follow-up of approximately 17 months. The median treatment duration was longer with carfilzomib, dexamethasone, and daratumumab vs carfilzomib and dexamethasone (70.1 vs 40.3 weeks). Grade 3 or higher adverse events were reported in 253 patients (82%) in the carfilzomib/dexamethasone/daratumumab group and 113 patients (74%) in the carfilzomib/dexamethasone group. The frequency of adverse events leading to treatment discontinuation was similar in both groups (carfilzomib, dexamethasone, and daratumumab, 69 [22%]; carfilzomib, dexamethasone 38 [25%]).
Clinical Implications: Carfilzomib, dexamethasone, and daratumumab significantly prolonged progression-free survival and led to deeper responses in patients with relapsed or refractory multiple myeloma when compared with carfilzomib and dexamethasone. Improvement was particularly meaningful in the subset of patients who were refractory to lenalidomide, a group in much need of a therapeutic advance.
Clinical Trial: Phase II, DREAMM-2 trial—Efficacy and safety of two different doses of belantamab mafodotin in patients with multiple myeloma who have failed prior therapy including an anti-CD38 monoclonal antibody, a proteasome inhibitor, and an immunomodulatory agent (NCT 03525678)8
Background: Patients with relapsed or refractory multiple myeloma represent an unmet clinical need. Belantamab mafodotin-blmf is a first-in-class antibody-drug conjugate that delivers a cytotoxic payload, monomethyl auristatin F to B-cell maturation antigen–expressing myeloma cells.
Methods: Patients with multiple myeloma whose disease failed to respond to prior therapy including an anti-CD38 monoclonal antibody, a proteasome inhibitor, and an immunomodulatory agent received belantamab mafodotin at 2.5 mg/kg or 3.4 mg/kg intravenously, once every 3 weeks until disease progression or unacceptable toxicity.
Results: Between June 18, 2018, and January 2, 2019, 293 patients were screened and 196 included in the intention-to-treat population (97 in the 2.5-mg/kg cohort and 99 in the 3.4-mg/kg cohort). As of June 21, 2019 (the primary analysis data cutoff date), 30 of 97 patients (31%; 97.5% CI = 20.8%–42.6%) in the 2.5-mg/kg cohort and 34 of 99 patients (34%; Cl = 23.9%–46.0%) in the 3.4-mg/kg cohort achieved an overall response. About 73% of responders had a response duration of at least 6 months when receiving the recommended dose of 2.5 mg/kg. Adverse reactions in at least 20% of patients who received belantamab mafodotin were keratopathy, decreased visual acuity, blurred vision, nausea, pyrexia, infusion-related reactions, and fatigue.
Clinical Implications: Based on these data, the U.S. Food and Drug Administration granted accelerated approval to belantamab mafodotin-blmf for adult patients with relapsed or refractory multiple myeloma who have received at least four prior therapies.8-10 The recommended belantamab mafodotin-blmf dose is 2.5 mg/kg as an intravenous infusion over approximately 30 minutes once every 3 weeks. This FDA approval is particularly relevant given the paucity of effective agents in patients with triple-class refractory multiple myeloma.
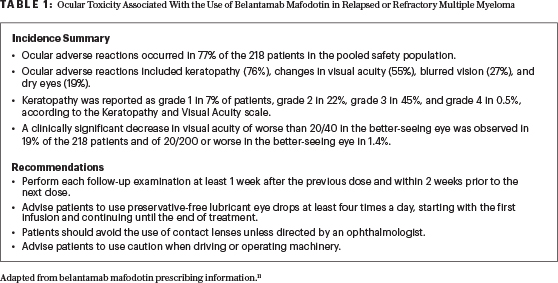
Belantamab mafodotin caused changes in the corneal epithelium, resulting in changes in vision (including severe vision loss and corneal ulcer) and symptoms such as blurred vision and dry eyes.9,10 It is FDA-approved under a Risk Evaluation and Mitigation Strategies program, which requires certification of prescribers and facilities and a detailed ophthalmologic examination prior to each dose (Table 1).11 A multidisciplinary approach involving close collaboration between ophthalmologists and hematologist/oncologists is central in treating patients with belantamab mafodotin. Future studies and longer follow-up will inform the reversibility of ophthalmologic toxicities and the potential for combination with other antimyeloma agent(s).
DISCLOSURE: Dr. Abutalib has served on advisory boards for AstraZeneca and Partner Therapeutics. Dr. Costa has received honoraria from Amgen, Celgene, and Janssen; has served as a consultant or advisor to AbbVie, Amgen, Celgene, and Karyopharm Therapeutics; has participated in a speakers bureau for Amgen and Sanofi; and has received research funding from Amgen and Janssen.
REFERENCES
1. Palladini G, Kastritis E, Maurer MS, et al: Daratumumab plus CyBorD for patients with newly diagnosed AL amyloidosis: Safety run-in results of ANDROMEDA. Blood 136:71-80, 2020.
2. Kastritis E, Palladini G, Minnema MC, et al: Subcutaneous daratumumab + cyclophosphamide, bortezomib, and dexamethasone in patients with newly diagnosed amyloid light chain amyloidosis: Primary results from the phase 3 ANDROMEDA study. 2020 European Hematology Association Virtual Congress. Abstract LB2604.
3. Palladini G, Sachchithanantham S, Milani P, et al: A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood 126:612-615, 2015.
4. Dimopoulos M, Quach H, Mateos MV, et al: Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): Results from a randomised, multicentre, open-label, phase 3 study. Lancet 396:186-197, 2020.
5. Palumbo A, Chanan-Khan A, Weisel K, et al; CASTOR investigators: Daratumumab, bortezomib, and dexamethasone for multiple myeloma N Engl J Med 375:754-766, 2016.
6. Dimopoulos MA, Moreau P, Palumbo A, et al: Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): A randomised, phase 3, open-label, multicentre study. Lancet Oncol 17:27-38, 2016.
7. Chari A, Martinez-Lopez J, Mateos MV, et al: Daratumumab plus carfilzomib and dexamethasone in patients with relapsed or refractory multiple myeloma. Blood 134:421-431, 2019.
8. Lonial S, Lee HC, Badros A, et al: Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): A two-arm, randomised, open-label, phase 2 study. Lancet Oncol 21:207-221, 2020.
9. Farooq AV, Degli Esposti S, Popat R, et al: Corneal epithelial findings in patients with multiple myeloma treated with antibody-drug conjugate belantamab mafodotin in the pivotal, randomized, DREAMM-2 study. Ophthalmol Ther. July 25, 2020 (early release online).
10. U.S. Food and Drug Administration: FDA granted accelerated approval to belantamab mafodotin-blmf for multiple myeloma. Available at https://www.fda.gov/drugs/drug-approvals-and-databases/fda-granted-accelerated-approval-belantamab-mafodotin-blmf-multiple-myeloma. Accessed September 1, 2020.
11. Blenrep (belantamab mafodotin-blmf) for injection, for intravenous use, GlaxoSmithKline, August 2020. Available at https://gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Blenrep/pdf/BLENREP-PI-MG.PDF. Accessed August 21, 2020.

