The ASCO Post is pleased to present Hematology Expert Review, an ongoing feature that quizzes readers on issues in hematology. In this installment, Drs. Abutalib and Landgren review the underlying data that shaped the updated International Myeloma Working Group (IMWG) diagnostic criteria for multiple myeloma and provide a clinical perspective on how best to include or exclude a diagnosis of multiple myeloma.

Syed A. Abutalib, MD

Ola Landgren, MD, PhD
“Appearances to the mind are of four kinds. Things either are what they appear to be; or they neither are, nor appear to be; or they are, and do not appear to be; or they are not, and yet appear to be. Rightly to aim in all these cases is the wise man’s task.”
—Epictetus
In 2014, the International Myeloma Working Group (IMWG) panel consensus1 updated 2003 criteria for the diagnosis of multiple myeloma by adding three new biomarkers, along with a few other modifications. Prior to the 2014 IMWG diagnostic criteria, the clinical paradigm for plasma cell dyscrasia without end-organ damage used to be “watch and wait,” and therapy was initiated once the patient was ill with end-organ damage. Certainly, one of the key limitations at the time the 2003 IMWG criteria were written was the scarcity of active agents (ie, therapy was mainly restricted to alkylating chemotherapy and steroids). Fortunately, the myeloma field has changed substantially, and in recent years, many effective and relatively less toxic agents have been approved by regulatory authorities worldwide.2
To adapt to such an overall favorable therapeutic environment, in addition to better understanding of the biology of the disease,3-6 in 2014, the updated IMWG diagnostic criteria1 were established with the addition of three diagnostic biomarkers to the previously described signs of a multiple myeloma diagnosis (ie, CRAB features—elevated calcium level, renal failure, anemia, and bone lesions). Specifically, these new diagnostic biomarkers include: (1) abnormal serum free light chain ratio (involved/uninvolved serum free light chain ratio) of ≥ 100 if involved serum free light chain is ≥ 10 mg/dL; (2) 60% or higher monoclonal plasma cell infiltration of the bone marrow or aspirate; and (3) two or more 5 mm or greater sized focal bone or bone marrow lesions, preferably on whole-body magnetic resonance imaging (MRI) scan (Table 1). The presence of any one of these diagnostic biomarkers with clonal bone marrow plasma cells ≥ 10% or biopsy-proven bony or extramedullary plasmacytoma, even in the absence of CRAB features, is now considered sufficient to diagnose multiple myeloma. Noteworthy, the cutoff values of these three described diagnostic biomarkers are arbitrarily defined from data reported in retrospective single-center studies.3-7 Subsequently, smaller efforts were launched to replicate these observations,8,9 and upon review of all these reports, the 2014 IMWG consensus group/committee thought it was clinically justifiable to integrate them into the diagnostic criteria.1

aBone marrow plasma cell percentage should preferably be estimated from a core biopsy specimen; in case of a disparity between the aspirate and the core biopsy, the highest value should be used.
bClonality should be established by showing κ/λ-light-chain restriction on flow cytometry, immunohistochemistry, or immunofluorescence.
Adapted from Rajkumar et al.1
FDG-PET-CT = 18F-fluorodeoxyglucose–positron-emission tomography–computed tomography; IMWG = International Myeloma Working Group; MRI = magnetic resonance imaging.
Clinical Vignette
A 55-YEAR-OLD African American woman is being evaluated for plasma cell disorder at her initial consultation. She has normal vital signs and physical examination. Medical and surgical histories are unremarkable. Chemistry laboratories are significant for an elevated total protein with low albumin levels, which prompts further testing. Serum protein electrophoresis and immunofixation show an M spike of 2.0 g/dL and immunoglobulin G (IgG) kappa, respectively. Serum free light chains ratio is 161.95, with kappa 1,260 mg/dL and lambda 7.78 mg/dL. The complete blood cell count and calcium levels are within normal laboratory range. Serum creatinine level is 0.76 mg/dL. The 24-hour urine has a monoclonal M spike without albuminuria. A whole-body skeletal survey, which was performed after denial of the 18F-fluorodeoxyglucose–positron-emission tomography–computed tomography (FDG-PET-CT) scan by the patient’s insurance carrier, is reported as “negative for bone lesion(s).” The results of bone marrow biopsy and aspirate specimens are anticipated to be reported in the next 2 days.
Question 1
Which skeletal imaging technique is suboptimal for detection of myeloma-defining bone lesion(s)?
A. Whole-body skeletal survey
B. Whole-body MRI
C. FDG-PET-CT scan
D. Low-dose whole-body CT
Expert Perspective
Correct answer: A. Whole-body skeletal survey has major sensitivity limitations when it comes to the detection of bone disease in clonal plasma cell dyscrasias; therefore, it is not recommended unless accessibility and resources to contemporary imaging technology are limited.1 The 2014 IMWG prefers FDG-PET-CT or low-dose whole-body CT or whole-body or spine MRI in all individuals with suspected smoldering multiple myeloma. The FDG-PET-CT scan has a sensitivity more than 20 to 30 times higher than that of a whole-body skeletal survey.10,11 In our experience, among 100 patients with smoldering multiple myeloma and a negative whole-body skeletal survey, we typically find 20 to 30 patients have multiple myeloma–defining bone lesions, usually by FDG-PET-CT scan.10
A common clinical question pertains to the use of FDG-PET-CT vs whole-body MRI: Is there added value in doing both? There are not enough data to give a definitive answer to this question; the 2014 IMWG criteria state that both methods are acceptable. In our clinical practice, we start with FDG-PET-CT scan; if it is negative for lytic bone lesions by the CT component, then we proceed with whole-body MRI—but only in cases where positive results would modify the management plan.
Question 2
Which statement about the FDG-PET-CT scan for detection of myeloma-defining bone lesion(s) is correct?
A. A PET component of FDG-PET-CT scan has no limitations for the diagnosis of plasma cell–associated bone lesion(s).
B. Hyperglycemia in the recipient may skew the results of the PET component of FDG-PET-CT scan, with false-positive plasma cell–associated bone lesions(s).
C. The routine review of the low-dose CT component of the FDG-PET-CT scan is crucial for optimal assessment of the skeleton for patients suspected of having plasma cell–associated bone lesion(s).
Expert Perspective
Correct answer: C. Increased uptake on the PET component of the FDG-PET-CT scan alone is not adequate for the diagnosis of plasma cell–associated bone lesions(s); evidence of underlying osteolytic bone destruction is required on the CT portion of the exam. Care should be taken to avoid over- or underestimation of equivocal or tiny lucencies seen on the CT component of the exam; if there are doubts about the nature of the abnormal bone lesion(s), it is reasonable to repeat the exam at a short interval (1 to 3 months; use clinical judgment), provided there are no other criteria to the diagnosis of multiple myeloma.1
In our practice, we utilize FDG-PET-CT as the default method to rule out lytic bone lesion(s) (by the CT component of the exam) in individuals suspected of having multiple myeloma. The PET component of the exam is nonspecific and nonsensitive for plasma cell–associated bone lesions(s).1 For example, it is not uncommon to have PET negativity due to the slow metabolism of the myeloma cells.10 False-negative exam results may be associated with hyperglycemia, recent administration of systemic steroids, or the presence of small subcentimeter bone lesion(s) in the skull. Moreover, PET positivity should also be questioned to rule out a false-positive reading (such as an artifact from previous bone metallic implants and ongoing/active physiologic muscle inflammation, infection, postoperative/bone biopsy area(s), and bone remodeling).1 This determination also requires close collaboration with the radiologist.
Quantification of abnormal standardized uptake values (“positive PET scan”) is currently not part of the routine clinical management for multiple myeloma; however, if deemed truly positive, then it could be followed as a clinical/functional biomarker of response to antimyeloma therapy.12 Two-way communication between the oncologist and the radiologist cannot be overemphasized. Lack of such communication may seed erroneous results and may compromise patient care.
Question 3
According to the 2014 IMWG criteria, which statement about MRI for detection of myeloma-defining bone lesion(s) is correct?
A. Two focal bone lesions, regardless of their size, and clonal bone marrow plasma cells > 10% are sufficient to diagnose multiple myeloma.
B. Two focal bone lesions, with predefined sizes, and clonal bone marrow plasma cells > 10% are sufficient to diagnose multiple myeloma.
C. Diffuse marrow signal pattern is sufficient to diagnose multiple myeloma.
Expert Perspective
Correct answer: B. A study by Kastritis and coworkers7 assessed 67 patients with smoldering multiple myeloma (as defined by older IMWG criteria) with MRI of the spine; 9 patients (14%) had 2 or more focal bone lesions. The median time to progression to multiple myeloma (as defined by older 2003 IMWG criteria) for such patients with more than 1 focal lesion was 15 months (95% confidence interval: 6–26 months). Over the period of 2 and 3 years, about 69% and 85% of patients, respectively, progressed to multiple myeloma. In contrast, the median time to disease progression for patients with 1 or no focal lesions was over 5 years (P < .001).
To expand on these findings, Hillengass and coworkers13 utilized whole-body MRI instead of MRI of the spine alone. In a series of 149 individuals with smoldering multiple myeloma (again, as defined by older 2003 IMWG criteria), they showed that, among patients with 2 or more focal lesions in the bone or marrow, 12 of 23 patients (50%) and 16 of 23 patients (70%) progressed to multiple myeloma within 13 months and 2 years, respectively.
Based on these two retrospective studies, the 2014 IMWG consensus panel decided to use spine or whole-body MRI as one of the three biomarkers to assist with the diagnosis of multiple myeloma. Currently, it is recommended to perform an additional imaging exam using low-dose CT or an FDG-PET-CT scan, even in patients with more than one focal lesion on MRI scan, if such lesions are < 5 mm or are equivocal. Of note, 5 mm or greater size on the bone and in the marrow is completely an arbitrary value.
Moreover, it should be stressed that there is no universal definition of a focal marrow lesion by MRI in the literature that defines multiple myeloma despite its firm standing (≥ 5 mm size) in the diagnostic criteria. Noteworthy, diffuse marrow infiltration pattern on MRI is associated with an increased risk of disease progression, but interestingly such findings if found, according to the 2014 IMWG criteria, do not qualify as one of the diagnostic criteria of multiple myeloma.
Although the 2014 IMWG criteria1 allow either whole-body or spine MRI due to differences in the availability of MRI around the globe, but intuitively less bone imaging or assessment may miss more multiple myeloma bone lesion(s); of note, approximately 10% of multiple myeloma bone lesions may be missed by isolated spine MRI.13
Question 4
According to the 2014 IMWG criteria, which statement defines renal insufficiency as a myeloma-defining event?
A. Fixed concentration of serum creatinine greater than 2.5 mg/dL.
B. Fixed concentration of serum creatinine greater than 1.8 mg/dL.
C. An estimated glomerular filtration rate of less than 40 mL/min.
Expert Perspective
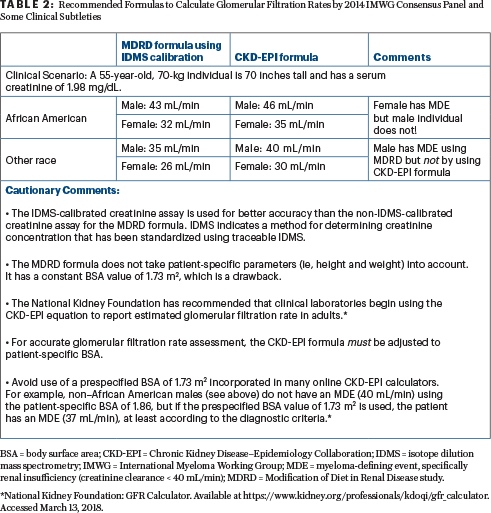
Correct answer: C. The 2014 IMWG criteria recommends that measured or estimated glomerular filtration rates (according to the modification of diet in renal disease or chronic kidney disease epidemiology collaboration formula) less than 40 mL/min (which correspond to about a 40% decrease from the lower limit of the normal glomerular filtration rate) be used instead of a fixed serum creatinine concentration to fulfill the criteria of renal insufficiency in the CRAB features. However, since not all institutions worldwide determine estimated glomerular filtration rate (preferred method), the 2014 IMWG criteria compromised, allowing serum creatinine > 2 mg/dL (173 mmol/L) as a marker of renal insufficiency as well. Additionally, are there data to support < 40 mL/ min is a better renal insufficiency “end-organ damage” criterion than < 45 mL/min or < 50 mL/min? The answer is No. These are not scientific arguments but rather clinical compromises (Formulas to calculate glomerulur filtration rates by 2014 IMWG Consensus Panel and some important clinical subtleties are available in Table 2).
Question 5
Which of the following disorder(s) with renal insufficiency may lead to misdiagnosis of multiple myeloma?
A. Light-chain Fanconi syndrome
B. Monoclonal gammopathy–associated membranoproliferative glomerulonephritis
C. Choices A and B are correct
Expert Perspective
Correct answer: C. Certain disorders such as amyloid light-chain amyloidosis, monoclonal immunoglobulin deposition disease, light-chain Fanconi syndrome, and monoclonal gammopathy–associated membranoproliferative glomerulonephritis are unique renal pathologic entities and may present with a concurrent clonal nonmyeloma-defining percentage of plasma cells (ie, < 60%) in the bone marrow. Such an occurrence may easily misguide the treating physician toward an inaccurate diagnosis of multiple myeloma or cast nephropathy. Although these disorders may occur in conjunction with multiple myeloma, in most individuals, such disorders occur independently, without evidence of other myeloma-defining event(s).1 It is important to stress that these disorders have clearly defined pathologic features, diagnostic criteria, prognosis, and therapy. Some investigators have collectively referred to these disorders under the term “monoclonal gammopathy of renal significance.”10
For this reason, patients with isolated (with absence of other “unexplained” myeloma-defining event[s]) renal insufficiency with clonal plasma cells in the marrow < 60% but > 10% should undergo diagnostic renal biopsy.11 However, there are few exceptions to this recommendation. First, if AL amyloidosis is suspected, we perform a renal biopsy once a fat pad biopsy is confirmed to be a “negative.” (A fat pad biopsy is less invasive than a renal biopsy.) Second, in the presence of high involved free light chains levels, typically > 1,500 mg/L, preemptive diagnosis of cast nephropathy (multiple myeloma) can be entertained, provided other causes of renal insufficiency are carefully excluded.
Question 6
Which statement about the diagnosis of multiple myeloma is correct?
A. Without CRAB features (calcium level elevated, renal failure, anemia, bone lesions), the diagnosis of multiple myeloma requires ≥ 50% bone marrow plasma cells.
B. CRAB features are always required to diagnose multiple myeloma.
C. Marrow sampling error or patchy marrow involvement may obscure a diagnosis of multiple myeloma.
Expert Perspective
Correct answer: C. Typically, patients with ≥ 60% (CD138 stain preferred over hematoxylin and eosin staining) infiltration (preferred over the bone marrow plasma cell) have concurrent end-organ damage (ie, CRAB features). However, there are cases of multiple myeloma where the bone marrow infiltration by plasma cells is ≥ 60% with an absence of CRAB features. Rarely (3%–5%) multiple myeloma is associated with bone marrow plasma cells of less than 10%, but in these circumstances, other prerequisites for diagnosis should be sought (Table 1).1
Marrow sampling error or patchy bone marrow involvement might obscure the diagnosis of multiple myeloma (collaboration with a hematopathologist is important), whereas in some rare cases, there are multiple plasmacytomas or lytic lesions with no generalized marrow involvement (macrofocal multiple myeloma); hence, the preference is the use of contemporary imaging techniques (over whole-body skeletal survey). “Myeloma-defining event(s)” cannot be attributed to multiple myeloma unless monoclonal bone marrow plasma cells are greater than 10% or histology-proven plasmacytoma is present. Equally important is to establish the clonality of plasma cells by demonstration of (1) κ/λ light-chain restriction by immunohistochemistry or immunofluorescence; (2) by demonstration of phenotypic clonality using flow cytometry; or (3) by immunoglobulin gene rearrangement studies to diagnose plasma cell dyscrasias accurately.
Question 7
Which statement about this individual’s case is correct?
A. It is best to repeat the serum and urine immunofixation studies to verify there are no laboratory error(s) while waiting for bone marrow plasma cell count by CD138 stain.
B. The patient should return in 6 months with repeat serum and urine immunofixation studies.
C. Antimyeloma therapy should be started immediately.
Expert Perspective
Correct answer: A. In the patient scenario presented, given the fact that this is a previously healthy, young woman who is completely asymptomatic and now presents with markedly abnormal free light chain ratio (> 100), it is not unreasonable to promptly repeat the serum and urine immunofixation studies to exclude laboratory error. This was done, and it was confirmed to be a true abnormality.
Summary
THE 2014 IMWG recommends implementation of “updated criteria for the diagnosis of multiple myeloma” in routine clinical practice and in clinical trials. Although the 2014 IMWG diagnostic criteria1 are not perfect, some of the limitations will be surmounted over time with the availability of more data, whereas others could potentially be tackled simply by regular and frequent collegial communication among medical professionals (oncologists, radiologists, hematopathologists, and often nephrologists) involved in the care of an individual patient.
Nevertheless, the intent and implications of the updated 2014 version are to facilitate earlier detection and earlier initiation of therapy. Future IMWG consensus criteria will benefit from participation of imaging specialists (eg, in MRI and FDG-PET-CT scans) in addition to myeloma specialists.
Acknowledgment: The authors would like to thank Arjun Patel, PharmD, BCOP, of Cancer Treatment Centers of America, Zion, Illinois, for review and critical feedback of this supplementary table.
DISCLOSURE: Drs. Abutalib and Landgren reported no conflicts of interest.
REFERENCES
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al: International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 15:e538-e548, 2014.
2. Ludwig H, Miguel JS, Dimopoulos MA, et al: International Myeloma Working Group recommendations for global myeloma care. Leukemia 28:981-992, 2014.
3. Landgren O: Multiple myeloma precursor disease: Current clinical dilemma and future opportunities. Semin Hematol 48:1-3, 2011.
4. Mailankody S, Mena E, Yuan CM, et al: Molecular and biologic markers of progression in monoclonal gammopathy of undetermined significance to multiple myeloma. Leuk Lymphoma 51:2159-2170, 2010.
5. Rajkumar SV, Larson D, Kyle RA: Diagnosis of smoldering multiple myeloma. N Engl J Med 365:474-475, 2011.
6. Larsen JT, Kumar SK, Dispenzieri, A, et al: Serum free light chain ratio as a biomarker for high-risk smoldering multiple myeloma. Leukemia 27:941-946, 2013.
7. Kastritis E, Moulopoulos LA, Terpos E, et al: The prognostic importance of the presence of more than one focal lesion in spine MRI of patients with asymptomatic (smoldering) multiple myeloma. Leukemia 28:2402-2403, 2014.
8. Leung N, Bridoux F, Hutchison CA, et al: Monoclonal gammopathy of renal significance. Blood 120:4292-4295, 2012.
9. Hutchison CA, Batuman V, Behrens J, et al: The pathogenesis and diagnosis of acute kidney injury in multiple myeloma. Nat Rev Nephrol 8:43-51, 2011.
10. Landgren O, Rajkumar SV: New developments in diagnosis, prognosis, and assessment of response in multiple myeloma. Clin Cancer Res 22:5428-5433, 2016.
11. Regelink JC, Minnema MC, Terpos E, et al: Comparison of modern and conventional imaging techniques in establishing multiple myeloma-related bone disease: A systematic review. Br J Haematol 162:50-61, 2013.
12. Moreau P, Attal M, Caillot D, et al: Prospective evaluation of magnetic resonance imaging and [18F]fluorodeoxyglucose positron emission tomography-computed tomography at diagnosis and before maintenance therapy in symptomatic patients with multiple myeloma included in the IFM/ DFCI 2009 trial: Results of the IMAJEM Study. J Clin Oncol 35:2911-2918, 2017.
13. Hillengass J, Fechtner K, Weber MA, et al: Prognostic significance of focal lesions in whole-body magnetic resonance imaging in patients with asymptomatic multiple myeloma. J Clin Oncol 28:1606-1610, 2010.

