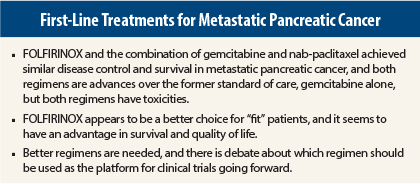
The choice between FOLFIRINOX (fluorouracil [5-FU], leucovorin, irinotecan, oxaliplatin) vs the combination of gemcitabine and nanoparticle albumin-bound (nab)-paclitaxel (Abraxane) for first-line treatment of metastatic pancreatic cancer is not much of a contest, judging by a discussion of “divergent views” at the recent 2014 Chemotherapy Foundation Symposium.
Speakers agreed that both regimens represent major advances over gemcitabine alone for the treatment of metastatic pancreatic cancer, providing almost identical disease control and similar survival. And they are associated with toxicities, but in the absence of a head-to-head randomized trial, FOLFIRINOX appears to be the standard of care for “fit” patients.
Howard Hochster, MD, of Yale University School of Medicine, New Haven, Connecticut, took the “pro” position for FOLFIRINOX, and Jordan Berlin, MD, of Vanderbilt-Ingram Cancer Center, Nashville, made the case for gemcitabine and nab-paclitaxel.1
Gemcitabine was established as the cornerstone of treatment in 1996. Subsequently, the four-drug combination FOLFIRINOX assumed that position when the PRODIGE 4/ACCORD 1 trial established it as the standard of care.2
The study randomized 342 patients with metastatic pancreatic cancer to receive FOLFIRINOX vs gemcitabine. The median overall survival was 11.1 months for FOLFIRINOX vs 6.8 months for gemcitabine (hazard ratio [HR] = .0.57, P > .001); the median progression-free survival was 6.4 months vs 3.3 months, respectively (P < .001); and the objective response rate was 31.6% vs 9.4%, respectively (P < .001).
More febrile neutropenia was reported in the FOLFIRINOX arm (5.4%). Patients in the FOLFIRINOX arm had less degradation in quality of life vs gemcitabine: 31% vs 66%, respectively (P < .001).
“This was the first study to show a benefit over gemcitabine and the first to achieve a median overall survival of 11.1 months in metastatic pancreatic cancer,” Dr. Hochster said.
Next, investigators at Yale University studied a modified version of FOLFIRINOX (mFOLFIRINOX), which reduced the dose of 5-FU and irinotecan by 25% in an attempt to cause fewer side effects, and compared results historically with gemcitabine and nab-paclitaxel.3 They found that the modified regimen did not compromise efficacy and improved the side-effect profile.
“Disease control and survival are identical with FOLFIRINOX and mFOLFIRINOX. Compared with gemcitabine and nab-paclitaxel, FOLFIRINOX is superior for parameters we measured, but it has slightly more diarrhea. Both regimens should be considered major treatment advances,” Dr. Hochster said.
“Either regimen can be used for first-line therapy. Survival and response seem better with FOLFIRINOX, as does quality of life. FOLFIRINOX should be standard of care for “fit” patients,” Dr. Hochster stated.
Gemcitabine and Nab-Paclitaxel
“There is probably not that much difference between these two regimens [FOLFIRINOX vs gemcitabine and nab-paclitaxel]. There may be some advantages for one or the other in selected patients, but we won’t see a randomized head-to-head comparison,” Dr. Berlin said.
Based on preclinical and early phase I/II studies, the phase III MPACT study randomized 842 patients to receive gemcitabine and nab-paclitaxel vs gemcitabine alone.4 The combination achieved superior response rates, progression-free survival, and overall survival compared with gemcitabine alone. The median overall survival was 8.5 months vs 6.7 months, respectively (P = .000015); the median progression-free survival was 5.5 months vs 3.7 months, respectively (P = .000024); the 1-year survival was 35% vs 22%, respectively; and the response rate was 23% vs 7%, respectively.
The main adverse events for the combination were fatigue and febrile neutropenia.
Comparing both regimens historically, the median overall survival favors FOLFIRINOX vs gemcitabine and nab-paclitaxel: 11.1 months vs 8.5 months, respectively.
There were some differences between MPACT, an international study, and PRODIGE, a French study, Dr. Berlin said. MPACT enrolled older, sicker patients, he said.
“Study sites can impact results. France is a modern country with good health-care systems, while resources are limited in Russia [one of the countries in the MPACT trial], and supportive measures are lacking,” he noted.
Neuropathy is seen with both regimens, so there is a problem in figuring out how to sequence them, he said.
No Real Winner
“We are nowhere near winning in this disease. Both regimens are losers—maybe that is a little strong, but they are not really winners. The bottom line is that we are not where we want to be. We have a long way to go in this disease,” Dr. Berlin said.
“Both regimens are reasonably toxic. The question is how do we go forward with use of biologics and sequencing,” Dr. Hochster commented. ■
Disclosure: Dr. Hochster reported no potential conflicts of interest.
References
1. Berlin J, Hochster H: Divergent views: FOLFIRINOX vs. gemcitabine-Abraxane. 2014 Chemotherapy Foundation Symposium. Presented November 6, 2014.
2. Conroy T, Desseigne F, Ychou M, et al: FOLIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817-1825, 2011.
3. Gunturu KS, Yao X, Cong X, et al: FOLFIRINOX for locally advanced and metastatic pancreatic cancer: Single institution retrospective review of efficacy and toxicity. Med Oncol 30:361, 2013.
4. Von Hoff DD, Ervin TJ, Arena FP, et al: Randomized phase III study of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic adenocarcinoma of the pancreas (MPACT). 2013 Gastrointestinal Cancers Symposium. Abstract LBA148.