What advanced basal cell carcinomas lack in frequency, they make up for in morbidity, and for these challenging patients, the hedgehog inhibitors have changed lives, according to experts at the 3rd Annual World Cutaneous Malignancies Congress, held recently in San Francisco.
“The majority of basal cell carcinomas are simple, but some become locally advanced and even metastasize. Traditionally, there was little we could do for these patients, but with understanding of the hedgehog pathway, we can now intervene,” said Aleksandar Sekulic, MD, PhD, Assistant Professor of Dermatology at the Mayo Clinic, Scottsdale, Arizona, who led the pivotal trial resulting in the approval of vismodegib (Erivedge).1
James MacDonald, MD, of Central Utah Clinic in Provo, added that the genetic profile is still being revealed. Nearly all sporadic basal cell carcinomas have mutations in two hedgehog pathway components—Patched (PTCH, a cell-surface transmembrane protein) and Smoothened (SMO, a G protein–coupled receptor protein)—which are targeted by hedgehog inhibitors, but the genetic hunt does not stop here.
“We have found that [basal cell carcinoma] is more than a molecular ‘one-trick pony,’” he said. “We used to think that the defects within the hedgehog signaling pathway were the primary or even the only driver in carcinogenesis. Now we know that there are other mutational defects—such as in TP53, which evolve over time and with ultraviolet exposure—that result in the various subtypes of basal cell carcinomas. The aggressive subtypes have a higher burden of these molecular defects.”
Hedgehog Inhibitors in the Pipeline
Currently, the focus remains on hedgehog inhibitors, since abnormal hedgehog signaling appears to be involved in most, if not all, basal cell carcinomas. “The approval of vismodegib and the potential for other agents to become available have changed our ability to treat this disease,” said Karl D. Lewis, MD, Associate Professor of Medicine at the University of Colorado, Denver.
The phase II ERIVANCE trial of vismodegib at 150 mg was recently updated by Dr. Sekulic at the 2014 ASCO Annual Meeting.2 The 30-month analysis showed a median progression-free survival of 9.3 months in metastatic patients and 12.9 months in patients with locally advanced disease; median duration of response of 14.8 months and 26.2 months, respectively; and median overall survival of 22.4 months in metastatic patients and not reached in the locally advanced cohort.
“Responses can be durable, and patients can be treated with vismodegib for quite some time. You can go years without a recurrence,” Dr. Lewis observed.
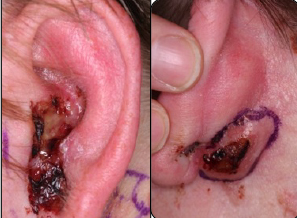
In development is a second hedgehog inhibitor, sonidegib, which produced equally impressive results in the phase II BOLT trial, first reported at the 2014 ASCO Annual Meeting and updated at the European Society for Medical Oncology (ESMO) 2014 Congress.3 The study met its primary endpoint of ≥ 30% objective response rate after a median follow-up of 13.9 months. With 200 mg (the dose for future trials), responses were observed at 12 months in 58% of patients with locally advanced basal cell carcinoma and in 8% with metastatic disease; the disease control rate was 91% and 92%, respectively, and median progression-free survival was 22 months and 13.1 months (Fig. 1).
The hedgehog inhibitors are also life-changing for patients with the challenging condition called basal cell nevus syndrome, also known as Gorlin syndrome. These patients may develop hundreds of basal cell carcinomas secondary to activation of target genes of the hedgehog pathway in cells that have lost both normal copies of PTCH.
“Vismodegib has been life-changing for Gorlin patients,” said Jean Y. Tang, MD, PhD, Associate Professor of Dermatology at Stanford School of Medicine in California. Dr. Tang co-led the U.S. randomized Gorlin study that tracked a total of 2,000 existing basal cell carcinomas and followed 694 new cases. “Patients randomly assigned to vismodegib had a remarkable reduction in new lesions,” she noted.
The real-world management of patients with advanced basal cell carcinoma and basal cell nevus syndrome is being captured in the RegiSONIC disease registry study, which will follow 750 patients from 75 sites. “We are asking how patients are being managed, can we treat them indefinitely [with hedgehog inhibitors], and can we interrupt treatment to manage side effects?” Dr. Tang said.
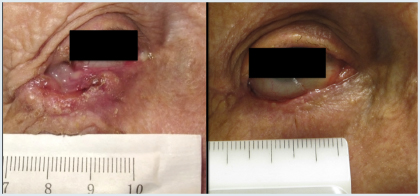
Neoadjuvant treatment might be the next clinical application of hedgehog inhibitors, according to Dr. Tang, whose open-label study of 15 patients with large basal cell carcinomas demonstrated the ability of vismodegib, given for 3 to 6 months, to reduce the anticipated surgical defect size by 27% (P = .006; Fig. 2).4 A randomized, double-blind study is evaluating this strategy.
Dealing With Toxicities
Drug-related toxicities are on-target effects of tissues that rely on the hedgehog signaling pathway. Common toxicities include muscle spasm, hair loss, taste abnormalities, and weight change. Sonidegib has also been shown to increase creatine kinase enzyme levels; to what degree this also occurs with vismodegib is not clear.
Axel Hauschild, MD, PhD, Head of the Skin Cancer Working Group at the University Hospital Schleswig-Holstein in Germany, commented that despite the adverse events, “Patients appreciate having an oral drug.” He noted that the number of patients being treated with vismodegib in Germany has been “much higher than expected.”
Dr. Lewis indicated that “the toxicity profile makes it difficult to maintain patients on treatment.” He said that while most toxicities are low-grade, “over the long term they wear on patients. I give dose holidays.”
In the U.S. randomized Gorlin study, 54% of patients receiving vismodegib discontinued drug treatment because of adverse events. Most patients who discontinue, however, choose to go back on the drug when their tumors return, Dr. Tang said. “The best management seems to be a short period of treatment interruption,” she suggested.
Intermittent dosing, therefore, is an important component of side-effect management, along with hydration and calcium, magnesium, and potassium supplementation, muscle relaxants, and calcium channel blockers, per the speakers.
Dr. Tang suggested that clinicians prepare patients for the likelihood of side effects. “When patients stop treatment, they continue to lose their hair for 2 months, but the hair grows back. You can’t avoid these side effects, so it’s best to prepare patients.” ■
Disclosure: Dr. Hauschild has received honoraria from and is a consultant for Amgen, BMS, Celgene, Eisai, GSK, Mediummune, MelaSciences, Merck Serono, MSD/Merck, Novaritis, Oncosec, and Roche Pharma. He has also received trial grants from Amgen, BMS, Celgene, Eisai, GSK, MelaSciences, Merck Serono, MSD/Merck, Novartis, Oncosec, and Roche Pharma. Dr. Lewis reported no potential conflicts of interest.
References
1. Sekulic A, Migden MR, Oro AE, et al: Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med 366:2171-2179, 2012.
2. Sekulic A, Migden MR, Basset-Seguin N, et al: Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: Final update (30-month) of the pivotal ERIVANCE BCC study. ASCO Annual Meeting. Abstract 9013. Presented June 2, 2104.
3. Drummer R, Guminski A, Gutzmer R, et al: Randomized, double-blind study of sonidegib (LDE225) in patients with advanced basal cell carcinoma. ESMO 2014 Congress. Abstract LBA33. Presented September 27, 2014.
4. Ally MS, Aasi S, Wysong A, et al: An investigator-initiated open-label clinical trial of vismodegib as a neoadjuvant to surgery for high-risk basal cell carcinoma. J Am Acad Dermatol. June 11, 2014 (early release online).