Children with acute lymphoblastic leukemia (ALL) considered at standard risk for relapse should continue to receive standard-intensity regimens, according to findings from the international randomized AIEOP-BFM ALL 2000 trial.1

Our study underlines the importance of well-designed, sufficiently powered studies for prospective evaluation of treatment reduction in childhood ALL—a major challenge for developing future therapy strategies.— Martin Schrappe, MD
Tweet this quote
A reduced-intensity treatment for children with ALL considered to have a favorable prognosis was associated with a higher relapse rate than was observed with the standard protocol, in the study presented at the Plenary Scientific Session of the American Society of Hematology (ASH) Annual Meeting & Exposition by Martin Schrappe, MD, of Christian-Albrechts University Kiel and University Medical Center Schleswig-Holstein in Germany.
“Any treatment modification in such patients with an excellent prognosis should be carried out with great caution, under controlled conditions,” Dr. Schrappe commented.
Dr. Schrappe noted that the study randomized “only the most favorable patients, selected by minimal residual disease response, and, unfortunately—especially in B-cell precursor ALL, the most common type—50% more relapses were observed in the less intensively treated patients.”
The study aimed to reduce the burden of chemotherapy with a lower-intensity delayed-intensification protocol, in order to spare short- and long-term toxicities, without compromising efficacy. The goal was to address the open question of the optimal—least toxic, most effective—treatment for childhood standard-risk ALL. The study evaluated a less intensive treatment for “well-selected” standard-risk patients, he said.
Study Design
From 2000 to 2006, the international randomized AIEOP-BFM ALL 2000 trial enrolled 4,741 children with ALL (age range, 1–17 years), of whom 1,164 were considered at standard risk for relapse. Standard risk was defined as minimal residual disease–negative status with two polymerase chain reaction markers for IgH or T-cell receptor gene rearrangements in week 5 after induction and in week 12 after consolidation. Marker sensitivity was at least 10-4, and the children had no clinical or biologic high-risk factors.
Reduced-Intensity Chemotherapy in Childhood ALL
- The international randomized AIEOP-BFM ALL 2000 trial evaluated whether a reduced-intensity treatment protocol could produce similar outcomes to standard-intensity chemotherapy in childhood ALL patients with favorable risk (minimal residual disease–negative, no high-risk features).
- After 8.6 years of follow-up, the less intensive regimen resulted in a 5-year event-free survival of 90.7% vs 94.7% with the standard regimen (P = .12).
- The 5-year event-free survival difference became statistically significant in the per-treatment analysis (90.6% vs. 94.9%; P = .041); trends favoring the standard approach were seen for cumulative incidence of relapse and overall survival.
- Differences were most notable among patients ≥ 10 years old and in those with B-cell precursor ALL.
The 1,164 standard-risk patients were randomized to standard-intensity delayed intensification with protocol II (P-II) or to the experimental, less intensive protocol III arm (P-III). P-III is shorter than P-II (duration, 29 vs 49 days), and its doses were reduced by 30% for dexamethasone, and by 50% for vincristine, doxorubicin, and cyclophosphamide, as compared to P-II. The intention was to prove noninferiority of the reduced-intensity treatment compared to standard treatment. The main endpoint was 4-year disease-free survival by per-protocol analysis.
“The aim was to reduce the treatment burden in the group of patients with best treatment response and lowest risk of relapse,” Dr. Schrappe said.
Key Findings
After a median follow-up of 8.6 years, the 5-year event-free survival rate was 90.7% in the reduced-intensity P-III arm vs 94.7% with the P-II regimen (P = .12), Dr. Schrappe reported.
“In the intent-to-treat analysis, there is not a statistically significant difference between the arms, even though there seems to be a trend for less intensively treated patients to do a little worse,” he observed. “Remember, we are talking about a patient group with an excellent prognosis—90% [survival] or more. It’s a different scenario with high-risk leukemia.”
He continued, “When you look at outcomes per treatment given, the picture is a little different. You see a significant impact when analyzed per treatment given.”
Significant differences or trends were observed, favoring the standard treatment approach, for event-free survival, cumulative incidence of relapse, and overall survival at 5 years (see Table 1).
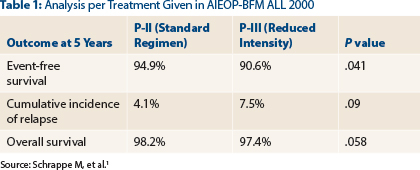
The difference in the B-cell precursor subtype was most striking, with 5-year event-free survival rates of 90.6% vs 95.2%, respectively (P = .043). In patients who were ETV6/RUNX1-negative, these rates were 87.7% and 94.2%, respectively (P = .027), Dr. Schrappe reported.
Furthermore, in the “critical age group” of patients aged 10 and older, “you especially see the disadvantage of reduced intensity,” he noted. In this group, 5-year event-free survival was 81.6% with P-III and 95.2% with P-II treatment (P = .037).
The incidence of death in remission was comparable, with five deaths on P-III and four on P-II. The number of grade 3/4 infections was lower in the acute phase of P-III therapy (4 vs 19), but in the late phase of dose intensification, there were actually more grade 3/4 infections with the reduced-intensity regimen (14 vs 7). Also unexpected, there were more second malignancies in the P-III arm (7 vs 4), he said.
Noting that some previous studies had suggested treatment could be deintensified without harm, he said his study shows that “only a randomized approach in a large cohort could detect differences that are significant and clinically relevant…. Our study underlines the importance of well-designed, sufficiently powered studies for prospective evaluation of treatment reduction in childhood ALL—a major challenge for developing future therapy strategies.” ■
Disclosure: Dr. Schrappe reported no potential conflicts of interest.
Reference


