The addition of the CD38 monoclonal antibody daratumumab to a standard regimen for patients with newly diagnosed transplant-eligible multiple myeloma significantly prolonged progression-free survival vs standard treatment in the phase III PERSEUS trial. The study was reported as a late-breaking abstract at the 2023 American Society of Hematology (ASH) Annual Meeting & Exposition by Pieter Sonneveld, MD, PhD, of the Erasmus MC Cancer Institute in Rotterdam, the Netherlands.1 The results were published simultaneously in The New England Journal of Medicine.2
The superior regimen in PERSEUS involved subcutaneous daratumumab plus bortezomib, lenalidomide, and dexamethasone (D-VRd) for induction and consolidation after autologous stem cell transplantation (ASCT), with daratumumab plus lenalidomide as maintenance therapy. Similar encouraging outcomes with D-VRd were also reported in a “real-world” experience at ASH 2023 by investigators from Emory University.

Pieter Sonneveld, MD, PhD
“The randomized, phase III results of PERSEUS support D-VRd followed by daratumumab/lenalidomide maintenance as a new standard of care for transplant-eligible patients with newly diagnosed multiple myeloma,” said Dr. Sonneveld.
Key Findings
At a median follow-up of 47.5 months, the 4-year progression-free survival rate in PERSEUS with D-VRd was 84.3% vs 67.7% with VRd alone, resulting in a 58% reduction in the risk of disease progression or death with the addition of daratumumab (hazard ratio [HR] = 0.42; P < .0001). D-VRd also significantly improved the depth of response, with two-thirds of those patients sustaining measurable residual disease (MRD) negativity for at least 1 year, Dr. Sonneveld reported.
The standard of care for transplant-eligible patients has been VRd followed by ASCT, VRd consolidation, and lenalidomide maintenance. Previous findings from the phase II GRIFFIN trial showed that the addition of daratumumab to VRd significantly improved progression-free survival.3 PERSEUS differed from GRIFFIN in that it was a much larger prospective randomized phase III trial, included daratumumab for maintenance, used subcutaneous rather than intravenous daratumumab and gave the drug every 4 weeks (to improve tolerability), and allowed maintenance daratumumab to be stopped if patients sustained MRD negativity for at least 12 months, Dr. Sonneveld explained. He considered this approach to be a “major step forward” as compared with other schedules with “less efficacy and more toxicity.”
The findings essentially confirm the practice of many myeloma specialists in the United States, said press briefing moderator Surbhi Sidana, MD, Assistant Professor of Medicine (Blood and Marrow Transplantation and Cellular Therapy) at Stanford University. “Many myeloma investigators were early adopters of D-VRd, but community hematologists may not be. GRIFFIN did show a progression-free survival benefit, but many people were holding out for results from a phase III study. This is very important confirmatory data that settle this issue.”

Surbhi Sidana, MD
Outside of the United States, where D-VRd is not commonly used, the results could be practice-changing, Dr. Sonneveld maintained.
Dr. Sidana also told The ASCO Post: “Many investigators and clinicians are still unsure if continued use of daratumumab (or another anti-CD38 monoclonal antibody) in maintenance is necessary, and this study did not answer that question. Many of us do not routinely use daratumumab in maintenance, except in high-risk patients. Additional ongoing studies including the SWOG S1803 study and the GMMG-HD7 study will answer that important question.”
About PERSEUS
The PERSEUS trial enrolled 709 adults with newly diagnosed, transplant-eligible multiple myeloma, randomly assigning them to:
Control arm: induction with bortezomib at 1.3 mg/m2 on days 1, 4, 8 and 11, plus lenalidomide at 25 mg on days 1 to 21, plus dexamethasone at 40 mg on days 1 to 4 and 9 to 12 (VRd), followed by ASCT. The same VRd schedule was given as consolidation therapy, which was followed by maintenance lenalidomide at 10 mg on days 1 to 28 until disease progression.
Investigational arm: VRd (same as above) plus 1,800 mg of subcutaneous daratumumab every 2 weeks (D-VRd), followed by transplant and then D-VRd consolidation with subcutaneous daratumumab at 1,800 mg given every 2 weeks. This was followed by maintenance with daratumumab at 1,800 mg every 4 weeks plus lenalidomide at 10 mg on days 1 to 28, until disease progression (guided by response and MRD status).
“I’d like to emphasize that we gave daratumumab as a subcutaneous infusion during induction and throughout maintenance, and we administered cycles of 28 days, making this easier for patients to tolerate. Finally, during maintenance, patients were allowed to stop daratumumab and continue with lenalidomide if after 24 months they were in a continuous complete response and had 12 months of sustained MRD negativity. These patients were followed very closely, and if they became MRD-positive again, they could resume the daratumumab,” Dr. Sonneveld said.
At a median follow-up of 47.5 months, the estimated progression-free survival at 48 months was 84.3% with D-VRd and 67.7% with VRd alone (HR = 0.42; P < .001). In the subgroup analysis, less benefit was observed among patients aged ≥ 65, but this group was relatively small and experienced few events, and the arms were imbalanced in terms of cytogenetic risk, he noted.
Several other secondary endpoints were also met, including response and MRD negativity (Table 1). Complete responses or better were observed with D-VRd regardless of sex, age, race, disease stage, type of multiple myeloma, cytogenetic risk, or performance status.
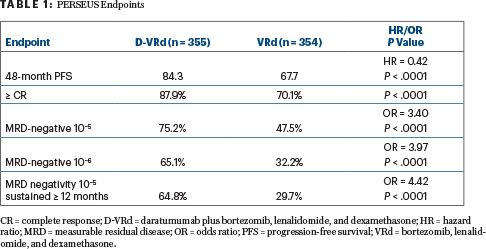
“We were not surprised to see the difference with the addition of daratumumab, but we were very surprised by the magnitude of the difference between the two arms,” Dr. Sonneveld commented. For MRD negativity, the large difference favoring D-VRd increased over time, “and this was even more evident for MRD at 10−6,” he noted, adding that although follow-up is short, the investigators believe an overall survival trend is emerging.
The observed safety profile was consistent with the known safety profiles for subcutaneous daratumumab and VRd.
DISCLOSURE: Dr. Sonneveld is a member of the Board of Directors or advisory committees for and/or has received research funding from Amgen, Bristol Myers Squibb, Celgene, Janssen, Karyopharm, and Pfizer. Dr. Sidana has received research funding and served on advisory boards for Janssen.
REFERENCES
1. Sonneveld P, Dimopoulos MA, Boccadoro M, et al: Phase 3 randomized study of daratumumab + bortezomib, lenalidomide, and dexamethasone (VRd) versus VRd alone in patients with newly diagnosed multiple myeloma who are eligible for autologous stem cell transplantation. 2023 ASH Annual Meeting & Exposition. Abstract LBA-1. Presented December 12, 2023.
2. Sonneveld P, Dimopoulos MA, Boccadoro M, et al: Daratumumab, bortezomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. December 12, 2023 (early release online).
3. Voorhees PM, Sborov DW, Laubach J, et al: Addition of daratumumab to lenalidomide, bortezomib, and dexamethasone for transplantation-eligible patients with newly diagnosed multiple myeloma (GRIFFIN): Final analysis of an open-label, randomised, phase 2 trial. Lancet Haematol 10:e825-e837, 2023.


