Patients with multiple myeloma now have access to an all-oral regimen, with the recent approval of the oral proteasome inhibitor ixazomib (Ninlaro) in previously treated patients. New pairings for the drug in relapsed/refractory and newly diagnosed patients are being studied, with investigators having reported their results at the 2015 American Society of Hematology Annual Meeting and Exposition.
One study (featured at a press briefing) combined ixazomib with lenalidomide (Revlimid) and dexamethasone—the regimen that recently received U.S. Food and Drug Administration (FDA) approval. The triplet significantly improved progression-free survival when compared with the doublet of lenalidomide/dexamethasone,1 reported Philippe Moreau, MD, of the University of Nantes in France.
“The all-oral regimen ixazomib/lenalidomide/dexamethasone may become a new standard of care for relapsed and refractory myeloma,” Dr. Moreau suggested.
Meletios A. Dimopoulos, MD, of the National and Kapodistrian University of Athens, Greece, described another all-oral, immunomodulatory drug–free regimen that included ixazomib, cyclophosphamide, and low-dose dexamethasone in newly diagnosed patients.2 And in patients progressing after lenalidomide, a regimen pairing ixazomib with pomalidomide (Pomalyst)/dexamethasone showed strong activity,3 according to Peter Voorhees, MD, of the University of North Carolina School of Medicine, Chapel Hill.
All-Oral Triplet in Tourmaline-MM1
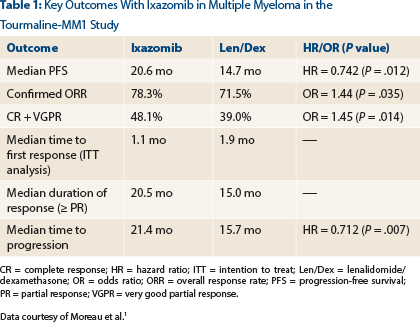
In the phase III Tourmaline-MM1 study, investigators from 26 countries enrolled 722 patients with relapsed/refractory myeloma. Patients had received one to three prior regimens, and 70% had prior exposure to a proteasome inhibitor. Patients were randomized to receive lenalidomide/dexamethasone or the same regimen plus ixazomib (4 mg on days 1, 8, 15) until disease progression or unacceptable toxicity.
After a median follow-up of 15 months, which was the time of the final progression-free survival analysis, median progression-free survival was 20.6 months with the triplet therapy vs 14.7 months in the control arm (hazard ratio [HR] = 0.742; P = .012), Dr. Moreau reported (Table 1).
“We saw a statistically significant, 35% improvement in progression with ixazomib,” he announced. “We also saw improved response rates, durable responses, and improved time to progression with ixazomib plus lenalidomide/dexamethasone.”
Benefit was as good in high-risk patients as in standard-risk patients—or perhaps better, he said, calling the drug’s impact “huge” for patients with t(4;14) and del(17p), who had a response rate of 78% and a reduction in risk of 41%.
“Ixazomib added limited additional toxicity to that seen with placebo and lenalidomide/dexamethasone,” Dr. Moreau noted. “There were low rates of peripheral neuropathy and no cardiovascular or renal signals. Patient-reported quality of life was maintained.”
Increased toxicity in the triplet arm was largely driven by low-grade events. “We saw no safety concerns. This triplet is a very safe combination,” he indicated.
The most common grade ≥ 3 toxicities were neutropenia, anemia, thrombocytopenia, and pneumonia. Gastrointestinal events included diarrhea, nausea, and vomiting. Peripheral neuropathy occurred in 27% of the ixazomib arm and 22% of the control arm; rash occurred in 36% and 23%, respectively.
Ixazomib/Cyclophosphamide/Dexamethasone
Dr. Dimopoulos reported findings from the phase II study of ixazomib/cyclophosphamide/low-dose dexamethasone (ICd) in 70 newly diagnosed patients.
“After a median follow-up of only about 9 cycles, an early objective response rate of 71% indicates that ICd is an active, all-oral immunomodulator-free proteasome inhibitor–based combination in front-line, elderly myeloma patients,” he said.
Patients were randomized to receive ixazomib, low-dose dexamethasone, and two different doses of cyclophosphamide (300 mg/m2 and 400 mg/m2), for up to 13 cycles (median 9), and then maintenance with single-agent ixazomib, and followed for a mean time of 7 months.
Preliminary results showed a best unconfirmed an overall rate of complete response plus very good partial response of 26%, and an early overall response rate of 71%. Responses were somewhat higher with the 300 mg/m2 dose of cyclophosphamide. Among patients with measurable M protein, 84% achieved at least a 50% reduction. At 12 months, progression-free survival is 80%.
“New responses are continuing to occur late in induction, and new at least very good partial responses occurring into maintenance suggest that increased responses may be expected as data mature,” Dr. Dimopoulos said.
Toxicity was manageable in both dose groups but was higher with the 400 mg/m2 dose of cyclophosphamide, he added. Serious adverse events were observed in 39% of patients receiving the lower dose and 50% with the higher dose.
“Research has shown that the combination of a proteasome inhibitor with cyclophosphamide and dexamethasone is active in patients with multiple myeloma. As treatment practices for multiple myeloma can vary across regions, it is important we gain an understanding of the utility of ixazomib in a number of combination settings,” Dr. Dimopoulos said. “Preliminary data suggest this may be a viable all-oral triplet.”
Ixazomib in Multiple Myeloma
■ The first oral proteasome inhibitor, ixazomib, has been paired with other agents in both relapsed/refractory and front-line settings in clinical trials. ■ The final progression-free survival analysis of the phase III TOURMALINE- MM1 showed a median progression-free survival of 20.6 months with ixazomib/lenalidomide/dexamethasone, vs 14.7 months with lenalidomide/ dexamethasone alone. ■ A phase II study demonstrated a response rate of 71% after 9 cycles of ixazomib/cyclophosphamide/low-dose dexamethasone in newly diagnosed patients not eligible for transplant. ■ The phase I Alliance A061202 study of refractory patients combined ixazomib with pomalidomide/dexamethasone, showed a response rate of 55%—rising to 100% among standard-risk patients.Ixazomib/Pomalidomide/Dexamethasone
Dr. Voorhees said the rationale is strong for a triplet containing ixazomib, pomalidomide, and dexamethasone in proteasome inhibitor–refractory disease. He cited strong preclinical data, the potential for synergy with the combination, and the potential for this triplet to combat clonal heterogeneity.
The phase I Alliance A061202 study enrolled 22 patients, of whom 32% were refractory to a lenalidomide/proteasome inhibitor combination and 68% of whom were refractory to the sequential use of these drugs. The majority of patients had high-risk cytogenetics.
“We found that pomalidomide, ixazomib, and dexamethasone can be combined safely,” Dr. Voorhees reported. “But moderate to severe hematologic toxicity is common and requires close monitoring and supportive care. Nonhematologic toxicity is less common and typically mild to moderate in severity.”
More than half the patients experienced grade 3/4 neutropenia, lymphopenia, and reductions in white blood cell count. A few patients had grade 3/4 anemia and thrombocytopenia. Peripheral neuropathy, rash, diarrhea, and other side effects were limited to grades 1/2.
“The preliminary efficacy of this combination is promising,” Dr. Voorhees reported. The response rate was 55% among patients refractory to both lenalidomide and a proteasome inhibitor, including 100% of standard-risk patients and 46% of high-risk patients.
“Responses were seen at all dose levels. Many of the responses have proven durable, even in the lower dose cohorts,” he added.
The study established a dose of 4 mg of pomalidomide and 3 mg of ixazomib as well tolerated. Enrollment into this dose level is ongoing. ■
Disclosure: Dr. Moreau received honoraria from and is an advisor to or a member of the board of directors for Bristol-Myers Squibb, Celgene, Millennium, Janssen-Cilag, and Novartis. Dr. Dimopoulos received honoraria from Amgen, Celgene, Janssen-Cilag, Novartis, Onyx, and Genesis. Dr. Voorhees received honoraria from Millennium/Takeda and is an advisor to or a member of the board of directors for Celgene.
References
- Moreau P, Masszi T, Grzasko N, et al: Ixazomib, an investigational oral proteasome inhibitor in combination with lenalidomide and dexamethasone, significantly extends progression-free survival for patients with relapsed and/or refractory multiple myeloma: The phase 3 Tourmaline-MM1 study. 2015 ASH Annual Meeting. Abstract 727. Presented December 7, 2015.
- Dimopoulos MA, Grosicki S, Jedrzejczak WW, et al: Randomized phase 2 study of the all-oral combination of investigational proteasome inhibitor ixazomib plus cyclophosphamide and low-dose dexamethasone in patients with newly diagnosed multiple myeloma who are transplant-ineligible. 2015 ASH Annual Meeting. Abstract 26. Presented December 5, 2015.
- Voorhees PM, Mulkey F, Hassoun H, et al: Alliance A061202, a phase I/II study of pomalidomide, dexamethasone and ixazomib versus pomlidomide and dexamethasone for patients with multiple myeloma refractory to lenalidomide and proteasome inhibitor based therapy: Phase I results. 2015 ASH Annual Meeting. Abstract 375. Presented December 6, 2015.