Findings from two major studies presented at the 2013 American Society of Hematology Annual Meeting not only confirm the benefit of rituximab (Rituxan) maintenance in follicular lymphoma, but also indicate that the longer the maintenance period, the greater the impact on progression-free survival. However, overall survival differences have still not emerged, even after 6 years of follow-up.
Longer Maintenance Doubles Remission Time
In a study designed to determine the optimal duration of rituximab maintenance in patients with follicular lymphoma, median progression-free survival doubled when treatment was continued to a maximum of 5 years, and this benefit of prolonged maintenance occurred “without increased undue toxicity,” said Christian J. Taverna, MD, of Kantonsspital in Munsterlingen, Switzerland.1
“Rituximab maintenance has been shown to be effective in patients with follicular lymphoma, but the optimal duration of maintenance therapy remains unknown,” he said.
The randomized phase III SAKK 35/03 trial, therefore, was initiated in 2004 to determine if maintenance with rituximab every 2 months for 5 years or until relapse or progression, unacceptable toxicity, or death would be superior to maintenance every 2 months for four treatments.
The study enrolled 270 patients with untreated, relapsed, stable, or chemotherapy-resistant follicular lymphoma (all grades) and treated them with four weekly doses of rituximab at 375 mg/m².
A total of 124 patients were chemotherapy-naive.
The 165 patients achieving a complete or partial response to this induction regimen were randomly assigned to receive rituximab at 375 mg/m² as either short-term maintenance (four administrations every 2 months) or long-term maintenance (every 2 months for a maximum of 5 years or until disease progression or unacceptable toxicity). The primary endpoint was event-free survival from the time of randomization. Events included disease progression or relapse, unacceptable toxicity, death from any cause, initiation of nonprotocol or concomitant steroids or radiotherapy, or secondary malignancy.
Primary Endpoint Not Met
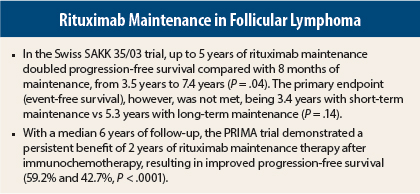
The primary endpoint, event-free survival, was not met with long-term maintenance rituximab. Median event-free survival was 3.4 years after short-term maintenance and 5.3 years with long-term maintenance, which under the prespecified log-rank test was not a statistically significant difference (P = .14), Dr. Taverna reported.
“We observed an imbalance in early events,” he said, chiefly an unexplained difference in disease progression and relapse during the first 8 months after randomization (3 in the short-term arm vs 10 in the long-term arm), when treatment in both arms was the same.
“This led to an early crossing of the event-free survival curves at 18 months,” he said, indicating this was responsible for the negative outcome.
In the short-term rituximab arm, three events occurred in the first 8 months, all disease progressions or relapses. In the long-term arm, 14 events occurred, including 1 death, 10 disease progressions or relapses, 2 secondary malignancies, and 1 unacceptable toxicity.
Nevertheless, progression-free survival, a secondary endpoint, was significantly improved with longer maintenance—from 3.5 years in the short-term arm to 7.4 years in the long-term arm (hazard ratio [HR] = 0.63; P = .04). Overall survival and response rates were similar in the two arms.
Importantly, in a retrospectively defined analysis of only patients at risk after 8 months from randomization, median event-free survival was significantly prolonged with longer maintenance: 7.1 years compared with 2.9 years (P = .004), Dr. Taverna reported.
At least one adverse event was experienced by 50% of patients in the short-term cohort and 76% in the long-term cohort. Maintenance was stopped due to unacceptable toxicity in three patients receiving long-term maintenance. Other adverse effects included, respectively, six and eight subsequent cancers and one and seven infections grade 3 and higher.
“There is no explanation for this difference in early events, when treatment in both arms was the same,” Dr. Taverna concluded. “Otherwise, outcomes were significantly better with long-term maintenance rituximab, including a doubling in median progression-free survival without an increase in toxicity.”
PRIMA Confirms Maintenance Benefit
Gilles Andre Salles, MD, PhD, of the Université Claude Bernard in Lyon, France, presented updated 6-year follow-up of the phase III PRIMA study of 2-year rituximab maintenance after immunochemotherapy, showing a 43% reduction in progression and a 38% reduction in time to next treatment, but no improvement in overall survival.
“The durability of rituximab maintenance benefit for progression-free survival and time to next treatment is consistent across patients with different Follicular Lymphoma International Prognostic Index (FLIPI) scores, chemotherapy induction regimens, and response to chemotherapy induction,” Dr. Salles reported.
PRIMA enrolled 1,217 patients treated with various rituximab-based induction regimens, then randomly assigned 1,018 to observation or rituximab maintenance. At 3 years, in the initial analysis, progression-free survival rates (from the time of randomization) were 75% for maintenance vs 58% for observation (hazard ratio [HR] = 0.55; P < .0001).
The updated analysis, with a median follow-up of 6 years, found progression-free survival rates to be 59.2% and 42.7%, respectively (HR = 0.57; P < .0001). At 70 months, subsequent treatment was required by 63.5% vs 51.0%, respectively (HR = 0.625; P < .0001), Dr. Salles reported. Overall survival was “excellent,” he said, at 88.7% and 87.4%, respectively (HR = 1.027; P = .885).
Subgroup Analyses
Benefit for maintenance was observed after either induction regimen: rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or rituximab plus cyclophosphamide, vincristine, and prednisone (R-CVP). “However, [the benefit associated with] R-CHOP may be a little more significant,” he suggested, citing a progression-free survival rate of 62.9% with maintenance vs 44.5% with observation (HR = 0.538; P < .0001).
By FLIPI score, FLIPI-low patients had the best outcomes, with a 76% progression-free survival rate vs 60% with observation (HR = 0.596; P = .0052), but all FLIPI groups benefited from maintenance over observation.
Two years of maintenance was beneficial regardless of response status after induction, with hazard ratios of 0.520 for complete responders, 0.635 for unconfirmed complete responders, and 0.449 for partial responders. Response to second-line treatment was 79% in the observation group and 76% in the maintenance group, and rates of histologic transformation were also similar, about 20% per arm.
“Rituximab maintenance did not lead to the selection of more aggressive clones in these patients,” Dr. Salles said. About half of all deaths were associated with lymphoma progression in each arm.
Disclosure: Dr. Taverna reported no potential conflicts of interest. Dr. Salles is a consultant for and has received honoraria and research funding from Roche.
References
1. Taverna CJ, Martinelli G, Hitz F, et al: Rituximab maintenance treatment for a maximum of 5 years in follicular lymphoma: Results of the randomized phase III trial SAKK 35/03. 2013 ASH Annual Meeting. Abstract 508. Presented December 9, 2013.
2. Salles GA, Seymour JF, Feugier P, et al: Updated 6 year follow-up of the PRIMA study confirms the benefit of 2-year rituximab maintenance in follicular lymphoma patients responding to frontline immunochemotherapy. 2013 ASH Annual Meeting. Abstract 509. Presented December 9, 2013.