In the Clinic provides overviews of novel oncology agents, addressing indications, mechanisms, administration recommendations, safety profiles, and other essential information needed for the appropriate clinical use of these drugs.
On December 3, 2014, blinatumomab (Blincyto) was granted accelerated approval for use in treating Philadelphia chromosome–negative relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).1,2
Supporting Trial
Approval was based on results of a single-arm trial in 185 patients showing achievement of durable complete remission/complete remission with partial hematologic recovery. Blinatumomab was administered by continuous infusion for 4 weeks of a 6-week cycle. In the first cycle, the initial dose was 9 µg/d for week 1, then 28 µg/d for the remaining 3 weeks. The target dose of 28 µg/d was administered in cycle 2 and subsequent cycles starting on day 1 of each cycle.
Among treated patients, the median age was 39 years (range, 18–79 years), 34% had undergone hematopoietic stem cell transplantation prior to receiving blinatumomab, and 17% had received more than two prior salvage therapies.
Complete remission/complete remission with partial hematologic recovery within two cycles of treatment, the primary endpoint, was achieved in 77 patients (41.6%, 95% confidence interval [CI] = 34.4%–49.1%), including complete remission in 60 patients (32.4%, 95% CI = 25.7%–39.7%). The majority of responses (62/77, 81%) occurred within cycle 1 of treatment.
Minimal residual disease response (< 104 on polymerase chain reaction) occurred in 80% of patients with complete remission, 58.8% of those with complete remission/complete remission with partial hematologic recovery, and 75.3% of responders in both groups. Median durations of response were 6.7 months (range, 0.46–16.5 months) in patients with complete remission, 5.0 months (range, 0.13–8.8 months) in those with complete remission with partial hematologic recovery, and 5.9 months in both groups. The hematopoietic stem cell transplantation rate among those who achieved complete remission/complete remission with partial hematologic recovery was 39%.
How It Works
Blinatumomab is a bispecific CD19-directed CD3 T-cell engager (BiTE) that binds to CD19 expressed on the surface of cells of B-lineage origin and CD3 expressed on the surface of T cells. It activates endogenous T cells by connecting CD3 in the T-cell receptor complex with CD19 on benign and malignant B cells.
The agent mediates formation of a synapse between the T cell and the tumor cell, upregulation of cell adhesion molecules, production of cytolytic proteins, release of inflammatory cytokines, and proliferation of T cells, resulting in redirected lysis of CD19-positive cells.
How It Is Given
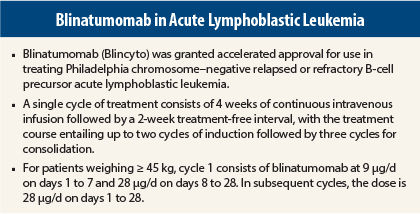
A single cycle of treatment consists of 4 weeks of continuous intravenous infusion followed by a 2-week treatment-free interval. For patients weighing ≥ 45 kg, cycle 1 consists of blinatumomab 9 µg/d on days 1 to 7 and 28 µg/d on days 8 to 28. In subsequent cycles, the dose is 28 µg/d on days 1 to 28. A treatment course consists of up to two cycles of induction followed by three cycles for consolidation.
Hospitalization is recommended for the first 9 days of the first cycle and the first 2 days of the second cycle. For all subsequent cycle starts and reinitiation (eg, if treatment is interrupted for at least 4 hours), supervision by a health-care professional or hospitalization is recommended.
Blinatumomab should be withheld for grade 3 cytokine-release syndrome and neurologic toxicity and for other grade 3 adverse reactions and restarted at 9 µg/d. It should be discontinued for grade 4 cytokine-release syndrome, neurologic toxicity, or occurrence of more than one seizure. Treatment discontinuation should be considered for other grade 4 adverse events.
As emphasized in the labeling, “It is very important that the instructions for preparation (including admixing) and administration … are strictly followed to minimize medication errors (including underdose and overdose).” The U.S. Food and Drug Administration approval of blinatumomab comes with a Risk Evaluation and Mitigation Strategy (REMS), consisting of a communication plan to inform health-care providers about the serious risks and the potential for preparation and administration errors.
Safety Profile
Safety was evaluated in 212 patients with B-cell precursor relapsed/refractory ALL who received at least one dose of blinatumomab. Patients had a median age of 37 years (range, 18–79 years), 63% were male, and 79% were white, 3% were Asian, and 3% were black or African American.
The most common adverse events of any grade were pyrexia (62%), headache (36%), peripheral edema (25%), febrile neutropenia (25%), nausea (25%), hypokalemia (23%), and constipation (20%). Adverse events of grade ≥ 3 occurred in 80% of patients, with the most common being “other pathogen” infection (25%), febrile neutropenia (23%), neutropenia (15%), anemia (13%), and bacterial infection (12%).
Serious adverse events occurred in 65% of patients, with the most common (≥ 2%) consisting of febrile neutropenia, pyrexia, pneumonia, sepsis, neutropenia, device-related infection, tremor, encephalopathy, infection, overdose, confusion, staphylococcal bacteremia, and headache.
Overall, neurologic toxicity occurred in approximately 50% of patients and was a frequent reason for interruption of therapy. Adverse events led to discontinuation of treatment in 18%, with the most common reasons including encephalopathy and sepsis. Adverse events led to death in 15% of patients, most commonly due to infection. No fatal adverse events occurred on treatment among patients in remission.
Blinatumomab carries boxed warnings for cytokine-release syndrome and neurologic toxicity, both of which may be severe, life-threatening, or fatal. It also carries warnings/precautions for infections, effects on ability to drive and use machines, and preparation and administration errors. ■
References
1. U.S. Food and Drug Administration: Blinatumomab. Available at www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm425597.htm. Accessed December 12, 2014.
2. Blincyto (blinatumomab) for injection prescribing information, Amgen Inc, December 2014. Available at www.accessdata.fda.gov/drugsatfda_docs/label/2014/125557lbl.pdf. Accessed December 12, 2014.
Report Adverse Events
Health-care professionals should report all serious adverse events suspected to be associated with the use of any medicine or device to FDA’s MedWatch Reporting System by completing a form online at www.fda.gov/medwatch/report.htm, by faxing (800-FDA-0178), by mailing the postage-paid address form provided online, or by telephone (800-FDA-1088).