Targeting one of the deadliest cancers and seeking to fill an unmet need, drug development in pancreatic cancer is an area of high interest. This was certainly the case at the 2015 Gastrointestinal Cancers Symposium, where results were impressive for some novel agents but disappointing for several others.
Hit: IMM-101
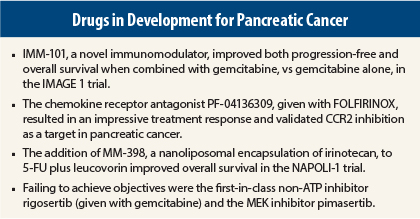
Given in combination with gemcitabine, the novel agent IMM-101 nearly doubled median overall survival time, vs gemcitabine alone, in an international randomized phase II study of 110 previously untreated patients with advanced pancreatic cancer.1
IMM-101 is a bacterially derived systemic immunomodulator given intradermally. In the IMAGE 1 trial, patients received up to 15 injections of IMM-101 over a maximum period of 12 cycles alongside gemcitabine.
The combination was associated with consistent and significant improvements in overall survival and progression-free survival, in both the intent-to-treat and per-protocol analyses. Improvements were most notable in the predefined subgroup of patients with metastatic disease (84% of the total). In the per-protocol analysis of this subgroup, median overall survival was 7.5 months, vs 4.4 months with gemcitabine alone (hazard ratio = 0.46, P = .002), and median progression-free survival was 4.4 months, vs 2.3 months (hazard ratio = 0.40, P < .001), reported Angus G. Dalgleish, MD, St. George’s University of London.
These differences represent increases of 70% in overall survival and 91% in progression-free survival. Differences in the intent-to-treat analyses were also statistically significant in the metastatic subgroup, he said.
“This randomized, controlled, proof-of-concept study has exceeded its objectives, providing clear direction for the further development of IMM-101, in combination with chemotherapy, as a first-line treatment option for metastatic pancreatic cancer,” Prof. Dalgleish said. “No additional burden of adverse events above those related to chemotherapy or the underlying disease was observed,” he said. The study is ongoing.
Hit: PF-04136309
In combination with FOLFIRINOX (fluorouracil [5-FU], leucovorin, irinotecan, oxaliplatin), the novel chemokine receptor antagonist PF-04136309 resulted in an impressive treatment response and validated CCR2 inhibition in pancreatic cancer, said Andrea Wang-Gillam, MD, PhD, of Washington University, St. Louis.2
The dense microenvironment of pancreatic tumors is thought to contribute to chemotherapy resistance. Inflammatory monocytes are frequently found in these tumors and are associated with poor outcomes. PF-04136309 inhibits the chemokine receptor CCR2 on inflammatory monocytes in the tumor microenvironment, the investigators explained.
The study enrolled patients with borderline resectable or locally advanced pancreatic cancer who received FOLFIRINOX alone or with PF-04136309 for six cycles. Of 29 evaluable patients receiving the combination, 14 (48.3%) had tumor shrinkage of at least 30%. Sequential biopsies showed that protumoral cytokines in the microenvironment were upregulated with FOLFIRINOX alone but were suppressed with the combination.
Study discussant Laura Goff, MD, of Vanderbilt University Medical Center, Nashville, commented, “This is a remarkable tumor response without substantial additional toxicity.… PF-04136309 represents an exciting new option for further development in pancreatic cancer.”
Hit: MM-398
The addition of MM-398, a nanoliposomal encapsulation of irinotecan, to 5-FU/leucovorin was shown to improve overall survival in patients with metastatic pancreatic cancer previously treated with gemcitabine in an expanded prespecified per-protocol analysis of the NAPOLI-1 trial.3 In the per-protocol population, MM-398 plus 5-FU/leucovorin achieved a median overall survival of 8.9 months, vs 5.1 months with 5-FU/leucovorin alone (hazard ratio = 0.47, P = .018).
Miss: Dual Non-ATP Inhibitor Rigosertib
The combination of rigosertib plus gemcitabine failed to demonstrate an improvement in survival or response, compared with gemcitabine alone, in an international randomized study.4 Rigosertib is a first-in-class non-ATP inhibitor of the Ras binding site on downstream effector proteins, including Raf and PI3K, and has shown efficacy in preclinical models.
The randomized, open-label study included 160 patients with untreated metastatic pancreatic cancer, enrolled from 50 sites internationally. Patients received rigosertib (1,800 mg/m2 on days 1, 4, 8, 11, 15, and 18) plus gemcitabine (1,000 mg/m2 on days 1, 8, and 15 of a 4-week cycle) or gemcitabine alone (same schedule).
Median overall survival was 6.1 months for rigosertib/gemcitabine, vs 6.4 months for gemcitabine alone. Median progression-free survival was 3.4 months for both groups, according to Aaron J. Scott, MD, of the University of Colorado, Denver.
Partial responses were observed in 19% on the combination and 13% receiving gemcitabine alone. Mutational analysis was performed on 47 samples, of which 41 were positive for mutations (KRAS, PI3K, p53), but no differences in outcomes emerged by mutational status.
Miss: MEK Inhibitor Pimasertib
An international phase II trial of a MEK inhibitor in patients with previously untreated metastatic pancreatic cancer was also negative.5 The study, led by Eric Van Cutsem, MD, of University Hospitals Gasthuisberg and KU Leuven in Belgium, randomized 88 patients to receive pimasertib plus gemcitabine or gemcitabine alone.
There was no difference in median progression-free survival between the arms, and in a preliminary analysis, overall survival was also similar. Disease control was numerically higher with the combination (61.4% vs 45.5%), but the response rates were similar.
“Pimasertib plus gemcitabine did not improve outcomes for patients with pancreatic cancer, compared with gemcitabine alone, which is consistent with findings for other MEK inhibitors,” Dr. Van Cutsem said. “The primary endpoint was not met, and assessment of secondary endpoints suggests that there is no clinically meaningful difference between the treatment arms.” ■
Disclosure: Prof. Dalgleish owns stock or has other ownership interests in Celgene; has received honoraria from Bristol-Myers Squibb, Celgene, and TNI BioTech; has served as a consultant or advisor for Binor Pharma, Celgene, CureVac, Immodulon Therapeutics, Ipsen, Novartis, PRMA Consulting, Roche, and TNI BioTech; is on the speakers bureau of Bristol-Myers Squibb; has received research funding from Celgene, GW Pharma, Immodulon Therapeutics; and has received patents and royalties from Binor Pharma, Celgene, GW Pharma, TNI BioTech, and Vulsana. Dr. Wang-Gillam has served as a consultant or advisor for Merrimack and Pfizer; has received research funding from Aduro Biotech, EMD Serono, Halozyme Therapeutics, Merrimack, Millennium, OncoMed, Pfizer, and Prometheus. Dr. Scott reported no potential conflicts of interest. Dr. Van Cutsem has received research funding from Amgen, Bayer, Boehringer, Lilly, Novartis, Merck Serono, Roche, and Sanofi.
References
1. Dalgleish AG, IMAGE 1 trial investigators: IMAGE 1: A multicenter randomized, open-label, proof-of-concept, phase II trial comparing gemcitabine with and without IMM-101 in advanced pancreatic cancer. 2015 Gastrointestinal Cancers Symposium. Abstract 336. Presented January 16, 2015.
2. Wang-Gillam A, Nywening TM, Sanford DE, et al: Phase 1B study of FOLFIRINOX plus PF-04136309 in patients with borderline resectable and locally advanced pancreatic adenocarcinoma. 2015 Gastrointestinal Cancers Symposium. Abstract 338. Presented January 16, 2015.
3. Chen L-T, Von Hoff DD, Li C-P, et al: Expanded analyses of NAPOLI-1: Phase 3 study of MM-398 with or without 5-fluorouracil and leucovorin, versus 5-fluorouracil and leucovorin, in metastatic pancreatic cancer previously treated with gemcitabine-based therapy. 2015 Gastrointestinal Cancers Symposium. Abstract 234. Presented January 15, 2015.
4. Scott AJ, O’Neil BH, Gomes C, et al: A phase II/III randomized study to compare the efficacy and safety of rigosertib plus gemcitabine versus gemcitabine alone in patients with previously untreated metastatic pancreatic cancer. 2015 Gastrointestinal Cancers Symposium. Abstract 342. Presented January 16, 2015.
5. Van Cutsem E, Hidalgo M, Bazin I, et al: Phase II randomized trial of MEK inhibitor pimasertib or placebo combined with gemcitabine in the first-line treatment of metastatic pancreatic cancer. 2015 Gastrointestinal Cancers Symposium. Abstract 344. Presented January 16, 2015.