TISAGENLECLEUCEL IS an anti-CD19 chimeric antigen receptor (CAR) T-cell product approved by U.S. Food and Drug Administration to treat selected hematologic malignancies.1,2 To appreciate the clinical trial findings summarized here, from selected abstracts presented at the 2018 American Society of Hematology (ASH) Annual Meeting & Exposition, it is prudent to review the initial data that led to its approval.1,2 These data are summarized in Table 1.
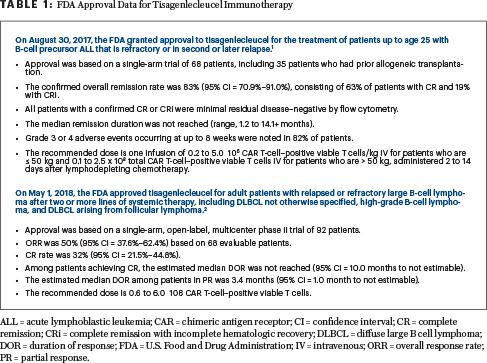
It is important to recognize that broader applicability of life-saving immunotherapy products continues to be limited, mainly due to logistic (eg, manufacturing slots) and financial (eg, hospitals and potential patients) barriers. Moreover, the in vivo immunologic effects and treatment durability remain an area of robust investigation. Ongoing areas of inquiry for these new therapies are highlighted in Table 2.

These questions, among others, are being tackled as our clinical and laboratory experiences grow simultaneously with these “living” CAR T-cell products.
ABSTRACT 895: Updated analysis of the efficacy and safety of tisagenlecleucel in pediatric (≥ 3 years) and young adults (≤ 21 years) with relapsed or refractory acute lymphoblastic leukemia (ALL)3
Rationale and Primary Study Endpoint: This is an update of the ELIANA trial, with additional patients and additional 11 months of follow-up. The primary endpoint was the overall remission rate (the rate of complete remission or complete remission with incomplete hematologic recovery) within 3 months of a single infusion.4
Key Findings: Among the 65 patients with complete remission and complete remission with incomplete hematologic recovery, 64 (98%) were minimal residual disease (MRD)-negative by flow cytometry within 3 months of therapy. Responses were ongoing in 29 patients; 19 patients relapsed before receiving additional anticancer therapy; 8 patients underwent hematopoietic cell transplantation while in remission, 8 received additional anticancer therapy (nontransplant); and 1 discontinued treatment while in remission. The probability of relapse-free survival at 18 months was 66% (95% confidence interval [CI] = 52%–77%). The median overall survival was not reached; the overall survival probability at 18 months was 70% (95% CI = 58%–79%).

Syed Ali Abutalib, MD

Bruce L. Levine, PhD
Cytokine-release syndrome occurred in 77% of study patients (grade 3 or 4; 48%; graded using the Penn scale5); 39% of patients received tocilizumab for treatment of cytokine-release syndrome with or without other anticytokine therapies; 48% of patients required intensive care unit–level care for cytokine-release syndrome. All cases of cytokine-release syndrome were reversible. Thirteen percent of patients experienced grade 3 neurologic events, with no grade 4 events or cerebral edema. A total of 25 postinfusion deaths were reported: 2 within 30 days; 23 after 30 days of infusion. See Table 1 for comparison of results of longer follow-up with initial approval.
Clinical Implications: The ELIANA study continues to confirm the efficacy of a single infusion of tisagenlecleucel without additional therapy. Tisagenlecleucel expansion in vivo correlated with cytokine-release syndrome severity, and persistence of tisagenlecleucel along with B-cell aplasia in the peripheral blood was observed for at least 2.5 years in some responding patients with relapsed or refractory ALL.
ABSTRACT 1684: Sustained disease control for adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL)6
Rationale and Study Endpoint: The updated analysis of the JULIET trial, with a median of 19 months of follow-up, offers an additional 5 months since the previous report.7 The primary endpoint was overall response rate (complete remission plus partial response).
Key Findings: At data cutoff (May 2018), 115 of the 167 patients were infused with a single dose of CAR T-cell–positive viable T cells. The median time from infusion to data cutoff was 19.3 months. At study entry, 77% of infused patients had stage III or IV disease, and 17% had double/triple-hit disease. A total of 55% and 43% had germinal center and activated B-cell molecular subtypes, respectively. All 99 patients in the main cohort had at least 3 months of follow-up or discontinued treatment earlier and were evaluable for efficacy.
The overall response rate was 54% (95% CI = 43%–64%), with 40% complete remissions and 13% partial responses. The overall response rate was consistent across prognostic subgroups (including those who had prior autologous transplant and double/triple-hit lymphoma). The median duration of response was not reached; the probability of being relapse-free was 66% (95% CI = 51%–78%) at 6 months and 64% (95% CI = 48%–76%) at 12 and 18 months. No relapses were observed beyond 11 months after infusion. No patients proceeded to allogeneic or autologous transplantation while in remission.
Grade 3 or 4 adverse events within 8 weeks of infusion included cytopenias lasting more than 28 days (34%); cytokine-release syndrome (23%, by the Penn scale6); infections (19%); febrile neutropenia (15%); neurologic events (11%); and tumor-lysis syndrome (2%). Three patients died within 30 days of infusion (all due to disease progression). No treatment-related mortality was reported. Since the previous report, no new deaths occurred due to causes other than disease progression. See Table 1 for comparison of results of longer follow-up with initial approval.
Clinical Implications: The results of this long-term follow-up show that tisagenlecleucel produced high and durable responses in a cohort of heavily pretreated adults with relapsed or refractory DLBCL.
ABSTRACT 2958: Safety and efficacy of tisagenlecleucel in patients with relapsed or refractory DLBCL and no evidence of active disease following bridging chemotherapy in the JULIET trial8
Study Rationale: Patients were enrolled in the JULIET trial based on local assessment of measurable disease at screening. To control disease during the period between apheresis and tisagenlecleucel infusion, 92% of the enrolled patients received bridging chemotherapy of the treating physician’s choice. Based on historical data, bridging chemotherapy is unlikely to provide sustained clinical response with conventional treatments. Eight patients in the JULIET trial achieved complete remission and had no measurable disease remaining by positron-emission tomography following bridging therapy and received tisagenlecleucel. It is unknown whether CAR T cells expand or provide clinical benefit in patients without detectable disease before tisagenlecleucel infusion.
Key Findings: Despite no measurable disease remaining before treatment with tisagenlecleucel, CAR T-cell expansion was observed in seven of eight patients, similar to the expansion in adults with active disease. Of the 8 patients, 5 remained on study and were progression-free more than 12 months after infusion, which is higher than would be estimated with bridging chemotherapy alone. Of the 8 patients, 5 experienced cytokine-release syndrome (2 grade 3, 3 grade 2, using the Penn scale5); 2 patients experienced neurologic events (both grade 1).
Clinical Implications: These data provide preliminary evidence that tisagenlecleucel may provide clinical benefit in patients with relapsed or refractory DLBCL who achieved complete remission with subsequent therapies and for design of CAR T-cell trials as consolidative therapy.
ABSTRACT 899: Tisagenlecleucel: Evaluation of in vivo CAR transgene levels in relapsed or refractory pediatric and young adult patients with ALL and adult patients with DLBCL9
Study Rationale: To determine whether CAR quantitative polymerase chain reaction measurements are associated with or predictive of response, CAR transgene levels and the timing of peak levels were examined. For those with ALL, 79 were from the ELIANA trial and 58 were from the ENSIGN trial; for patients with DLBCL, 93 patients were from the JULIET trial.
Key Findings: In both patients with ALL and DLBCL, CAR transgene was initially detected at high levels, with high variability in both responders and nonresponders. Although the majority of responding patients tended to have persistent transgene levels, some patients maintained favorable clinical responses despite a lack of quantifiable transgene.
Clinical Implications: The results indicate that quantitative polymerase chain reaction testing for CAR transgene in the blood of tisagenlecleucel-treated patients should not be used for making treatment decisions. In addition, the quantitative polymerase chain reaction measurements in peripheral blood do not reflect on the trafficking of CAR T-cell–positive cells to sites outside the peripheral blood, particularly the bone marrow. Also, further data are needed to improve our understanding of how CAR transgene blood levels relate to disease burden and the duration of response and whether this information is clinically useful. ■
Dr. Abutalib is Associate Director, Hematology and BMT Program; Director, Clinical Apheresis Program, Cancer Treatment Centers of America, Zion, Illinois; Associate Professor, Roseland Franklin University of Medicine and Science; as well as Founder and Co-Editor, Advances in Cell and Gene Therapy. Dr. Levine is Barbara and Edward Netter Professor in Cancer Gene Therapy; Founding Director, Clinical Cell and Vaccine Production Facility Center for Cellular Immunotherapies; Deputy Director, Technology Innovation and Assessment, Department of Pathology and Laboratory Medicine, Perelman School of Medicine, Abramson Cancer Center University of Pennsylvania, Philadelphia.
DISCLOSURE: Dr. Abutalib is an advisor for AstraZeneca Pharmaceuticals. Dr. Levine has stock and other ownership interest in Tmunity Therapeutics and Brammer Bio; has received honoraria from Novartis, Terumo, CRC Oncology, and Cure Genetics; is a consultant/advisor for Brammer Bio, Incysus, Ori Biotech, and Avectas; a patent or intelluctual property interest with the University of Pennsylvania licensed to Novartis and licensed to Tmunity Therapeutics.
REFERENCES
1. U.S. Food and Drug Administration: FDA approves tisagenlecleucel for B-cell ALL and tocilizumab for cytokine release syndrome. Available at www.fda. gov/drugs/informationondrugs/approveddrugs/ucm574154.htm. Accessed January 18, 2019.
2. U.S. Food and Drug Administration: FDA approves tisagenlecleucel for adults with relapsed or refractory large B-cell lymphoma. Available at www. fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm606540.htm. Accessed January 18, 2019.
3. Grupp SA, Maude SL, Rives S, et al: Updated analysis of the efficacy and safety of tisagenlecleucel in pediatric and young adult patients with relapsed/ refractory acute lymphoblastic leukemia. 2018 ASH Annual Meeting & Exposition. Abstract 895. Presented December 3, 2018.
4. Maude SL, Laetsch TW, Buechner J, et al: Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 378:439-448, 2018.
5. Porter D, Frey N, Wood PA, et al: Grading of cytokine release syndrome associated with the CAR T cell therapy tisagenlecleucel. J Hematol Oncol 11:35, 2018.
6. Schuster SJ, Bishop MR, Tam C, et al: Sustained disease control for adult patients with relapsed or refractory diffuse large B-cell lymphoma: An updated analysis of JULIET, a global pivotal phase 2 trial of tisagenlecleucel. 2018 ASH Annual Meeting & Exposition. Abstract 1684. Presented December 1, 2018.
7. Borchmann P, Tam CS, Jäger U, et al: An updated analysis of JULIET, a global pivotal phase 2 trial of tisagenlecleucel in adult patients with relapsed or refractory diffuse large B-cell lymphoma. 2018 Congress of the European Hematology Association. Abstract S799. Presented June 16, 2018.
8. Bishop MR, Maziarz RTT, Waller EK, et al: Safety and efficacy of tisagenlecleucel treatment in patients with relapsed/refractory diffuse large B-cell lymphoma and no evidence of active disease following bridging chemotherapy in the JULIET trial. 2018 ASH Annual Meeting & Exposition. Abstract 2958. Presented December 2, 2018.
9. Awasthi R, Mueller KT, Yanik GA, et al: Evaluation of in vivo CAR transgene levels in relapsed/refractory pediatric and young adult ALL and adult DLBCL tisagenlecleucel-treated patients. 2018 ASH Annual Meeting & Exposition. Abstract 899. Presented December 3, 2018.

