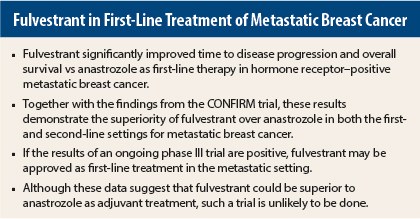
Fulvestrant (Faslodex) at a 500-mg dose was superior to anastrozole as first-line therapy for advanced hormone receptor–positive breast cancer in the phase II FIRST study.1 Overall survival and time to disease progression were significantly better with fulvestrant than with the current standard of care, anastrozole.
“This is an exciting and promising finding. Together with results from an earlier phase III trial in the second-line setting called CONFIRM, fulvestrant has now shown an advantage in the first- and second-line settings. I don’t know any other endocrine therapy where there is a time to disease progression and overall survival advantage in both the second- and first-line settings,” said lead author John Robertson, MD, of Graduate Entry Medicine and Health School, University of Nottingham, Royal Derby Hospital, United Kingdom.
Fulvestrant, a selective estrogen–receptor downregulator, is given via intramuscular injection once a month. The drug is approved for use in the second-line setting for metastatic hormone receptor–positive breast cancer that has progressed on first-line hormonal therapy. It is not approved as adjuvant therapy, even though it is considered the most potent antiendocrine therapy available, Dr. Robertson noted.
At a press conference during the 2014 San Antonio Breast Cancer Symposium, Dr. Robertson explained that fulvestrant was developed at a dose of 250 mg, and at that dose, it was found to be equivalent to anastrozole and similar to tamoxifen.
“Then it was decided that the dose of fulvestrant could be doubled in the second-line setting. The CONFIRM study showed that the higher dose significantly improved the time to disease progression and overall survival,” he continued.
Prolonged Control of Disease
In the FIRST trial, fulvestrant (500-mg dose) was compared with anastrozole in the first-line setting in 205 patients with advanced or metastatic breast cancer. The primary endpoint was clinical benefit rate, as reflected by disease control at the end of 6 months of treatment; this endpoint was achieved in 72.5% of the fulvestrant group vs 67% of the anastrozole group, demonstrating that fulvestrant at the 500-mg dose was at least as effective as anastrozole on this measure.
Fulvestrant achieved significantly prolonged control of disease. At 13 months, the median time to disease progression was 23.4 months with fulvestrant vs 13 months with anastrozole, which represented a highly significant 34% reduction in the risk of disease progression (P = .01).
Overall survival was not defined as an endpoint until the protocol was amended in 2011. At a median follow-up of 49.6 months for fulvestrant and 42.5 months for anastrozole, 23 patients (22.5%) were alive in the fulvestrant group, and 10 patients (9.7%) were alive in the anastrozole group. The median overall survival was 54.1 months for fulvestrant vs 48.4 months for anastrozole (P = .041), an absolute increase of 5.7 months. In addition, the overall survival benefit was consistent across all subgroups.
“To put these results in context, 30 years ago, the average survival in hormone receptor–positive metastatic breast cancer was 24 months. We have improved survival step by step,” Dr. Robertson stated.
No new safety concerns for either drug emerged during the trial.
‘Could Be Practice-Changing’
Dr. Robertson said the FALCON phase III study was initiated to support the regulatory approval of fulvestrant as first-line therapy in advanced breast cancer. If it is positive for fulvestrant, “this study could be practice-changing,” he noted.
Dr. Robertson said, “The data cry out for an adjuvant study. But this is a commercial issue, and adjuvant studies take many years to do. The drug is going to go off patent soon. It is unlikely that the company will do an adjuvant trial.”
Press conference moderator C. Kent Osborne, MD, Director of the Dan L. Duncan Cancer Center at Baylor College of Medicine in Houston, noted, “Because fulvestrant was not better than tamoxifen in a study of first-line treatment of metastatic disease (albeit with the lower 250-mg dose), it was not taken into an adjuvant therapy trial. Now that the higher dose has shown to be better than the aromatase inhibitor anastrozole, an adjuvant trial is reasonable.” n
Disclosure: Dr. Robertson reported receiving consulting fees for advisory boards organized by AstraZeneca; honoraria for speaking at non–Accreditation Council for Continuing Medical Education events; and serving as an investigator in trials sponsored by AstraZeneca. In addition, all funding related to these research studies has been paid to his academic institution, and Dr. Robertson received travel expenses to make a presentation on the FALCON study at the 2013 San Antonio Breast Cancer Symposium.
Reference
1. Robertson JFR, Llombart-Cussac A, Felti D, et al: Fulvestrant 500 mg versus anastrozole as first-line treatment for advanced breast cancer: Overall survival from the phase II ‘FIRST’ study. 2014 San Antonio Breast Cancer Symposium. Abstract S6-04. Presented December 12, 2014.