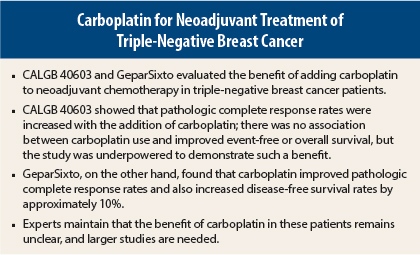
Studies presented at the 2015 San Antonio Breast Cancer Symposium built upon an increasing body of data in support of the neoadjuvant use of carboplatin in patients with triple-negative breast cancer. Overall, however, the studies fell short of establishing carboplatin’s role in this malignancy and left oncologists still uncertain as to when and how to incorporate the drug.
The studies were the phase II CALGB/Alliance 40603 trial, which showed improvements in pathologic complete response rates with carboplatin,1 and the phase II German GeparSixto trial, which showed improved pathologic complete response rates and demonstrated a benefit for carboplatin in event-free survival.2
CALGB 40603 Details
This study evaluated carboplatin administered every 3 weeks at an area under the curve [AUC] of 6 and bevacizumab (Avastin) at 10 mg/kg as a component of neoadjuvant chemotherapy for triple-negative breast cancer. A previous analysis showed that the addition of these agents to weekly paclitaxel followed by dose-dense doxorubicin and cyclophosphamide significantly improved pathologic complete response rates in the breast, and carboplatin also improved pathologic complete responses in the breast and axilla.3
The current analysis evaluated the association between pathologic complete response and event-free and overall survival among 443 patients followed for a median of 39 months. The study population had a 3-year event-free survival rate of 74% and a 3-year overall survival rate of 83%.
William Sikov, MD, Associate Professor of Medicine at Brown University, Providence, Rhode Island, reported that pathologic complete response was achieved in the breast by 52% and in the breast/axilla by 47%. Achievement of pathologic complete response or residual cancer burden class 1 was observed in 60%.
The achievement of pathologic complete response was associated with risk reductions of approximately 70% for event-free survival and up to 80% for overall survival. These results are consistent with the meta-analysis by Cortazar et al showing a 76% reduction in events and 84% reduction in mortality when patients with triple-negative disease achieve a pathologic complete response,4 Dr. Sikov pointed out.
The 3-year event-free survival rate was 86% for patients achieving pathologic complete response in the breast/axilla, vs 62% for those who did not (P < .0001). The 3-year overall survival rate was 93% and 73%, respectively (P < .0001). These improved outcomes, however, could not be specifically tied to treatment with carboplatin or bevacizumab, Dr. Sikov reported.
“Despite significantly higher [pathologic complete response] rates seen in CALGB 40603, neither carboplatin nor bevacizumab has yet been shown to improve relapse-free survival or overall survival when administered as part of neoadjuvant therapy in stage II to III triple-negative breast cancer,” he said. He acknowledged, however, that the study was underpowered to make this determination.
“Our results support the value of [pathologic complete response] as a good surrogate for long-term outcomes on a patient level,” he said, but evidence remains lacking that pathologic complete response can be used as a surrogate for event-free or overall survival “on a trial level.”
GeparSixto Details
“GeparSixto supports the use of carboplatin as part of neoadjuvant treatment in all patients with triple-negative breast cancer,” according to Gunter von Minckwitz, MD, Chair of the German Breast Group in Neu-Isenburg and Professor of Gynecology at the University Women’s Hospital in Frankfurt, Germany.
GeparSixto involved 595 patients with triple-negative or HER2-positive breast cancer, randomly assigned to neoadjuvant therapy with weekly paclitaxel and liposomal doxorubicin, with or without weekly carboplatin (AUC 2, later reduced to AUC 1.5). The triple-negative subset also received concurrent bevacizumab, while those with HER2-positive tumors received trastuzumab (Herceptin) and lapatinib (Tykerb).
The primary endpoint, pathologic complete response in all patients, was not improved by the addition of carboplatin: 43.7% with carboplatin vs 36.9% without (P = .107). However, the benefit was clear in the triple-negative subgroup, whose pathologic complete response rates improved from 37% to 53% with carboplatin (P = .005), Dr. von Minckwitz reported.
“Carboplatin improved disease-free survival substantially in patients with triple-negative breast cancer,” he noted. In this subgroup, disease-free survival was 85.8% with carboplatin and 76.1% without (hazard ratio = 0.56, P = .0350). The drug showed no effect in patients with HER2-positive breast cancer.
“The disease-free survival effect of carboplatin was correctly predicted by its extensive effect on [pathologic complete response], supporting the surrogacy of [pathologic complete response],” he added.
GeparSixto also demonstrated a strongly positive effect for carboplatin on pathologic complete response and disease-free survival in patients with wild-type BRCA1/2, 50.8% of whom achieved a pathologic complete response with carboplatin, compared to 33.1% who did not (P = .005).
“BRCA wild-type patients who did not get carboplatin had worse disease-free survival. The effect of carboplatin was mostly seen in wild-type patients, and this was a surprise,” he said. There were only 50 patients with BRCA mutations, which may have diminished the power to show an effect in this group, he acknowledged.
“Favorable prognosis after [pathologic complete response] was confirmed and was independent of germline BRCA status,” he concluded. ■
Disclosure: Dr. Sikov is on the speakers bureau for Eisai and is on advisory boards for Celgene and AbbVie. Dr. von Minckwitz disclosed relationships with Roche, Teva, and GlaxoSmithKline.
References
1. Sikov WM, Berry DA, Perou CM, et al: Event-free and overall survival following neoadjuvant weekly paclitaxel and dose-dense AC +/- carboplatin and/or bevacizumab in triple-negative breast cancer: Outcomes from CALGB 40603
(Alliance). 2015 San Antonio Breast Cancer Symposium. Abstract S2-05. Presented December 9, 2015.
2. von Minckwitz G, Loibl S, Schneeweiss A, et al: Early survival analysis of the randomized phase II trial investigating the addition of carboplatin to neoadjuvant therapy for triple-negative and HER2-positive early breast cancer (GeparSixto). 2015 San Antonio Breast Cancer Symposium. Abstract S2-04. Presented December 9, 2015.
3. Sikov WM, Berry DA, Perou CM, et al: Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer:
CALGB 40603 (Alliance). J Clin Oncol 33:13-21, 2015.
4. Cortazar P, Zhang L, Untch M, et al: Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 384:164-172, 2014.