At the 2014 ASCO Annual Meeting, one phase III trial confirmed the promise of a novel agent in advanced multiple myeloma, while another cooperative group trial returned some rather surprising results in newly diagnosed myeloma patients.
Panobinostat Doubles Response, Prolongs Remission
The phase III PANORAMA 1 trial demonstrated clear benefit for adding the investigational agent panobinostat to bortezomib (Velcade) in previously treated advanced multiple myeloma. The results were reported by Paul G. Richardson, MD, Clinical Disease Program Leader and Director of Clinical Research, Jerome Lipper Multiple Myeloma Center, Dana-Farber Cancer Institute, Boston.1
“The primary endpoint was met (P < .0001), with a clinically relevant increase in median progression-free survival of approximately 4 months,” Dr. Richardson announced.
Panobinostat is an oral pan–histone deacetylase (HDAC) inhibitor whose actions ultimately result in cell-cycle arrest and apoptosis. One of the drug’s key targets is HDAC6, an enzyme that is an important target in myeloma, as well as others in the HDAC family. In preclinical studies, the combination of panobinostat plus bortezomib shows synergy, as panobinostat inhibits the aggresome pathway of protein degradation that is upregulated when the proteasome pathway is inhibited by bortezomib.
PANORAMA 1 was an international randomized, double-blind, placebo-controlled phase III trial that enrolled 768 patients who had received one to three prior lines of treatment but were not bortezomib-refractory. More than half the patients had received a prior autologous stem cell transplant.
Patients were randomly assigned to panobinostat or placebo, each in combination with intravenous bortezomib (as subcutaneous bortezomib was not yet approved at the time of the trial’s initiation) and dexamethasone. Responders after eight cycles went on to receive less frequent dosing of bortezomib/dexamethasone.
After a median follow-up of 28 months, the combination significantly improved progression-free survival, from 8.1 months with placebo to 12.0 months, a 37% highly significant reduction in risk (P < .0001), which Dr. Richardson called a “clinically relevant benefit.”
While the overall response rate was similar between the arms—60.7% with panobinostat and 54.6% with placebo (P = .087)—the combination conveyed a near-doubling in complete and near-complete responses—27.6% vs 15.7%, respectively (P = .00006). The panobinostat arm also improved the median duration of response (13.1 vs 10.9 months), median time to response (1.5 vs 2.0 months), and median time to progression (12.7 vs 8.5 months).
Median overall survival, however, was not significantly different between the arms: 33.6 months in the panobinostat arm and 30.4 months in the placebo arm (P = .87), although the analysis remained early and the data immature for this key endpoint.
“These results confirm the promising efficacy of panobinostat, bortezomib, and dexamethasone previously observed in the PANORAMA 2 trial with patients who were both heavily pretreated and also bortezomib-refractory,” Dr. Richardson said.
The most common adverse event with the combination arm was thrombocytopenia, which was grade 3/4 in 67.4% with panobinostat and 31.4% with placebo, but was both generally manageable and reversible. Other grade 3/4 adverse events occurring more frequently with the HDAC inhibitor included lymphopenia, neutropenia, diarrhea, and asthenia/fatigue; however, these led to discontinuation of the drug in fewer than 5% of patients. Deaths deemed possibly treatment-related by the investigator numbered 11 (2.9%) in the panobinostat arm and 7 (1.9%) in the control arm.
“The adverse events were predictable and generally manageable with supportive measures and dose reduction,” he said.
Other panobinostat-containing combinations and additional HDAC inhibitors are currently in clinical trials, with promising results to date, Dr. Richardson noted. Moreover, the use of subcutaneous bortezomib may further improve the therapeutic index of the combination, and trials are either underway or planned to confirm this, he added.
Lenalidomide No Better Than Thalidomide
Results of the E1A06 trial, presented by A. Keith Stewart, MBChB, Professor of Medicine at the Mayo Clinic, Scottsdale, Arizona, were met with some surprise, as thalidomide (Thalomid) performed as well as lenalidomide (Revlimid) in combination with melphalan/prednisone in newly diagnosed patients ineligible for high-dose therapy.2
The study enrolled 306 patients (median age, 76), randomly assigning them to melphalan/prednisone plus either 12 cycles of thalidomide (100 mg), with thalidomide as maintenance (MPT-T), or lenalidomide (10 mg), with lenalidomide as maintenance (MPR-R). The study had a noninferiority design with a superiority alternative. The median follow-up was 40.7 months.
“The findings were statistically inconclusive,” Dr. Stewart said. “The study did not prove inferiority or noninferiority of MPR-R compared to MPT-T.”
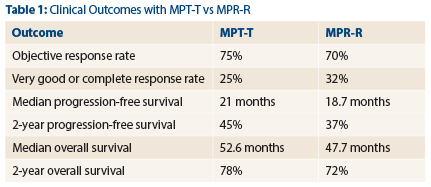
The response rates, depth of response, time on therapy, progression-free survival rates and overall survival rates for the two regimens were similar, Dr. Stewart reported (see Table 1).
For MPT-T and MRP-R, respectively, response rates were 75% and 70%, very good or complete response rates were 25% and 32%, median progression-free survival was 21 months and 18.7 months, and 2-year progression-free survival was 45% and 37%.
The finding that thalidomide performed as well as lenalidomide was apparently not explained by differences in drug exposure. Median time on therapy was 12 months, with no differences between the arms. Both groups received approximately 80% of the planned dose and 46% started maintenance. Patients receiving maintenance treatment had a median time on therapy of 23 months, which was also not different between groups.
“But overall toxicity was less with MPR-R,” Dr. Stewart noted. Grade ≥ 3 overall toxicity was 73% with MPT-T vs 58% with MPR-R (P = .007), and nonhematologic toxicity was 40% vs 59%, respectively (P = .001). “Patient-reported quality of life was also better with MPR-R at the end
of induction.”
Dr. Stewart pointed out that the survival outcome for the thalidomide arm is similar to those seen in previous studies of this regimen. However, the results for the lenalidomide arm were less impressive than those previously reported: In the MM 05 trial, for example, median progression-free survival was 31 months for MPT-R, and median overall survival was
56 months.
Dr. Stewart also put the results into the context of current treatment. “In the United States, melphalan-based regimens are now seldom utilized due to the availability of lenalidomide and bortezomib in newly diagnosed patients,” he noted. ■
Disclosure: Dr. Richardson is a consultant/advisor for Celgene, Johnson & Johnson, Millennium, and Novartis. Dr. Stewart is a consultant/advisor for Celgene. For full disclosures of all study authors, visit meetinglibrary.asco.org.
References
1. Richardson PG, Hungria VT, Yoon S, et al: Panorama 1. ASCO Annual Meeting. Abstract 8510. Presented June 2, 2014.
2. Stewart AK, Jacobus SJ, Fonseca R, et al: E1A06. ASCO Annual Meeting. Abstract 8511. Presented June 1, 2014.