ASCO recently released a clinical practice guideline update on ovarian suppression as part of the extant guideline on adjuvant endocrine therapy in hormone receptor–positive breast cancer, and the recommendations were summarized in the June 10, 2016, issue of The ASCO Post. Also in this issue, Katarzyna J. Jerzak, MD, FRCPC, and Kathleen I. Pritchard, MD, FRCPS, of Sunnybrook Odette Cancer Centre, University of Toronto, offered their perspective on the implications of this 2016 ASCO Guideline for Clinical Practice.
The 2016 ASCO Clinical Practice Guideline, “Update on Ovarian Suppression” (updated from “Guideline on Ovarian Ablation in the Treatment of Premenopausal Women With Early-Stage Invasive Breast Cancer”), uses only the term “ovarian suppression” (with LHRH [luteinizing hormone-releasing hormone] agonists) in the recommendations and in all the discussion.1-3 The focus of the updated guideline is clearly on the question of combined ovarian function–targeting plus endocrine therapies. Additionally, under Guideline Implementation, the first sentence states: “ASCO guidelines are developed for implementation across health settings.” However, by dropping any reference to ovarian ablation plus endocrine therapy, the updated guideline not only ignores a long-standing approved therapy, but also is inconsistent with the Guideline Implementation statement.
Various Perspectives
The perspective by Drs. Jerzak and Pritchard states: “High-quality evidence to support a recommendation for bilateral oophorectomy as opposed to reversible ovarian suppression with an LHRH agonist is lacking.” The authors follow this statement by noting that such evidence from the SOFT and TEXT trials has not been reported. However, the E-3193 INT-0142, begun over 20 years ago, and the SOFT and TEXT trials, begun about 15 years ago—which offered ovarian ablation as equivalent alternatives to LHRH agonist treatment—could not have been judged scientifically and ethically acceptable in the United States and many other countries if high-quality evidence regarding equivalence of these approaches had not been assessed to be present.
There are several perspectives whose application to the issue of guidelines for treatment supports a conclusion that ovarian ablation (surgical oophorectomy) plus tamoxifen is a better treatment than LHRH agonist plus tamoxifen. To begin, in its report on “Shaping the Future of Oncology: Envisioning Cancer Care in 2030,” the ASCO Board of Directors identified three key drivers of change that will have the biggest impact on cancer care over the coming decades: big data, cancer panomics, and delivering value.4
Although focused in the United States, the ASCO Conceptual Framework to Assess the Value of Cancer Treatment Options states that ASCO’s “highest priority” is delivery of high-quality care to all patients with cancer and discusses “financial toxicity” and the desire of patients for financial information.5 Its value framework for assessment of adjuvant treatments considers clinical benefit, toxicity, and cost.
The Institute of Medicine (IOM) proposes that to decrease the use of wasteful and unethical health services, quality of care must be assessed at multiple levels: effectiveness, safety, efficiency, patient-centeredness, timeliness, and equitability.6
For surgical oophorectomy plus tamoxifen and LHRH plus tamoxifen, in Table 1 are summarized comparative data for the ASCO and IOM measures of quality of care.7-11 In aggregate, these data clearly show the superiority of surgical oophorectomy plus tamoxifen.
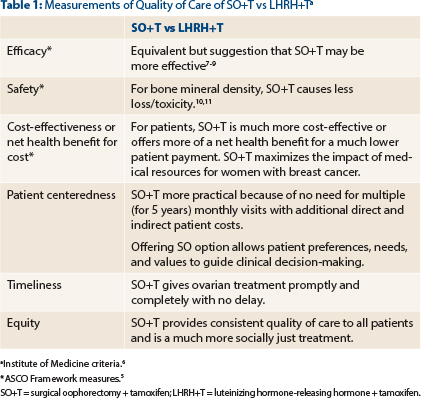
In the context of these circumstances and statements, which emphasize the equivalence in efficacy of ovarian ablation and LHRH agonist treatments as well as the commitment to value and all global cancer victims, I believe there is a mismatch between the values and practice embodied in the updated guideline.
Surgical oophorectomy plus tamoxifen is the optimal adjuvant endocrine treatment for premenopausal women with hormone receptor–positive tumors and should be so recommended in the ASCO guideline. ■
—Richard R. Love, MD
Madison, Wisconsin
References
1. Burstein HJ, Lacchetti C, Griggs JJ: Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline update on ovarian suppression summary. J Oncol Pract 12:390-393, 2016.
2. Burstein HJ, Lacchetti C, Anderson, et al: Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline update on ovarian suppression. J Clin Oncol 34:1689-1701, 2016.
3. Griggs JJ, Somerfield MR, Anderson H, et al: American Society of Clinical Oncology endorsement of the Cancer Care Ontario practice guideline on adjuvant ovarian ablation in the treatment of premenopausal women with early-stage invasive breast cancer. J Clin Oncol 29:3939-3942, 2011.
4. ASCO Board of Directors: Shaping the Future of Oncology: Envisioning Cancer Care in 2030: Outcomes of the ASCO Board of Directors Strategic Planning and Visioning Process, 2011-2012. Available at http://www.asco.org/sites/new-www.asco.org/files/content-files/about-asco/documents/2030-shaping-future-oncology-cancer-care.pdf. Accessed July 7, 2016.
5. Schnipper LE, Davidson NE, Wollins DS, et al: American Society of Clinical Oncology statement: A conceptual framework to assess the value of cancer treatment options. J Clin Oncol 33:2563-2577, 2015.
6. Institute of Medicine: Crossing the Quality Chasm: A New Health System for the 21st Century. March 2001. Available at http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2001/Crossing-the-Quality-Chasm/Quality%20Chasm%202001%20%20report%20brief.pdf. Accessed July 7, 2016.
7. Love RR, Laudico AV, Van Dinh N, et al: Timing of adjuvant surgical oophorectomy in the menstrual cycle and disease-free and overall survival in premenopausal women with operable breast cancer. J Natl Cancer Inst 107:djv064, 2015.
8. Love RR: Adjuvant surgical oophorectomy plus tamoxifen in premenopausal women with operable hormone receptor-positive breast cancer: A global treatment option. Clin Breast Cancer. March 31, 2016 (early release online).
9. Love RR, Hossain SM, Hussain MM, et al: Luteal versus follicular phase surgical oophorectomy plus tamoxifen in premenopausal women with metastatic hormone receptor-positive breast cancer. Eur J Cancer 60:107-116, 2016.
10. Love RR, Young GS, Laudico AV, et al: Bone mineral density changes following surgical oophorectomy and tamoxifen adjuvant therapy for breast cancer. Cancer 119:3746-3752, 2013.
11. Gnant MF, Mlineritsch B, Luschin-Ebengreuth G, et al: Zoledronic acid prevents cancer treatment-induced bone loss in premenopausal women receiving adjuvant endocrine therapy for hormone-responsive breast cancer: A report from the Austrian Breast and Colorectal Cancer Study Group. J Clin Oncol 25:820-828, 2007.

