Eribulin (Halaven), a cytotoxic agent approved for advanced/metastatic breast cancer, may improve overall survival for patients with two common and difficult-to-treat forms of advanced/metastatic sarcoma, investigators reported at the 2015 ASCO Annual Meeting.1
Eribulin is a microtubule inhibitor that blocks cell division. In preclinical models, it also has been shown to impact tumor cells via vascular remodeling, reversal of epithelial-mesenchymal transition, as well as suppression of migration and invasion.
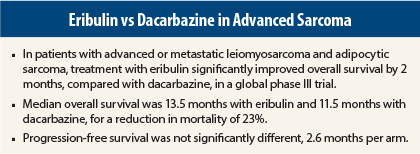
In the global phase III study (Study 309), eribulin reduced the risk of death by 23%, compared with dacarbazine, in patients with advanced liposarcomas and leiomyosarcomas, reported Patrick SchÖffski, MD, of University Hospitals Leuven in Belgium.
“Patients with advanced or metastatic disease have very poor outcomes, and their systemic treatment options are very limited,” he said. “The study’s primary endpoint of overall survival—a very reliable endpoint—was met. Eribulin had a favorable median survival of 13.5 months, vs the standard agent dacarbazine, 11.5 months (hazard ratio = 0.768; P = .0169).”
“The study population represents a high-risk group of patients with comorbid conditions, multiple prior drug regimens, and intermediate-to-high tumor grade, and therefore these results represent an important breakthrough. For me as a sarcoma oncologist, this is a clinically meaningful result, given the high unmet need in these rare, hard-to-treat diseases,” he said.
Study Details
Study 309 is a randomized, open-label multicenter phase III trial in which 452 patients with advanced leiomyosarcoma or adipocytic sarcoma (ie, liposarcoma) received eribulin (1.4 mg/m2 on days 1 and 8) or dacarbazine (850–1,200 mg/m2 on day 1) every 21 days until disease progression. More than 40% of patients had received more than two prior regimens.
The primary endpoint was overall survival, which was significantly improved in the eribulin cohort. Secondary endpoints, however, were not significantly different. Median progression-free survival was 2.6 months in both arms. The progression-free survival rate at 12 weeks was 33% for the eribulin arm and 29% for the dacarbazine arm.
The toxicity of eribulin was consistent with prior experience. The most common adverse events grade ≥ 3 with the drug were neutropenia (35%) and fatigue (3%). About one-third of patients experienced some degree of nausea, alopecia, and constipation. There were two treatment-related deaths in the eribulin arm.
Two ASCO experts commented on the study at a press briefing. Gary K. Schwartz, MD, noted, “There’s never been a randomized study of this type showing a survival benefit in advanced sarcoma in the history of medical oncology. It’s a small step forward in oncology but a major step for sarcoma patients.”
Press briefing moderator Don S. Dizon, MD, added, “This is going to be welcome news for patients with these two rare sarcoma types, especially since we understand this study population was very heavily pretreated. Active agents have not been readily available for them.” ■
Disclosure: Dr. SchÖffski has received honoraria from and served as a consultant or advisor to Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, GlaxoSmithKline, Iteos Therapeutics, Mundipharma, Novartis, Pique, Plexxikon, Prime Oncology, Servier, Swedish Orphan Biovitrum, Threshold Pharmaceuticals, and ThromboGenics. He is also on the speakers bureau of GlaxoSmithKline, Novartis, Prime Oncology, and Swedish Orpha Biovitrum. Drs. Schwartz and Dizon reported no potential conflicts of interest.
Reference
1. SchÖffski P, et al: 2015 ASCO Annual Meeting. Abstract LBA10502. Presented June 1, 2015.