More than 650 studies were presented at the 2014 Gastrointestinal Cancers Symposium, which attracted a multidisciplinary group of more than 3,500 medical, surgical, and radiation oncologists and gastroenterologists. The following briefs highlight a handful of noteworthy studies from the meeting.
Updated Survival Outcome in MPACT
An updated analysis of the MPACT trial (cutoff, May 2013) upheld the overall survival benefit of nab-paclitaxel (Abraxane) plus gemcitabine, vs gemcitabine alone, in metastatic pancreatic adenocarcinoma.1 Median overall survival was 8.7 months with the combination and 6.6 months with gemcitabine alone (hazard ratio [HR] = 0.72; P < .0001), and the 24-month overall survival rate was 10% vs 5%, respectively, according to David Goldstein, MD, Senior Staff Specialist in the Department of Medical Oncology at Prince of Wales Hospital in Sydney, Australia.
In the updated model, cancer antigen (CA) 19-9 was a significant predictor of overall survival and the number of metastatic sites was no longer significant. Treatment with nab-paclitaxel plus gemcitabine appeared to reduce the negative effect of CA 19-9 as a prognostic factor, he said.
“Longer follow-up demonstrated a median overall survival difference of 2.1 months and identification of patients surviving at least 3 years (4%) in the nab-paclitaxel plus gemcitabine arm,” Dr. Goldstein said. “We believe nab-paclitaxel is an important option for patients with metastatic pancreatic cancer, with the potential for long-term survivors.”
Circulating Tumor Cells in Pancreatic Cancer Diagnosis
Circulating tumor cells can aid in the diagnosis of pancreatic ductal adenocarcinoma and can identify patients with metastases at presentation, according to a prospective analysis from the University of California, Los Angeles.2 Researchers evaluated 61 consecutive patients suspected of having pancreatic cancer, or recently diagnosed with it but untreated. They examined 2 mL of blood for the presence and number of circulating tumor cells using NanoVelcro technology enhanced by anti-EpCAM enrichment.
Of the 61 patients, 41 had cancer and 20 had nonmalignant pathology on tissue biopsy. “We were able to discriminate between pancreatic ductal adenocarcinoma and non-adenocarcinoma diseases using [circulating tumor cell] presence as a marker,” said Jacob S. Ankeny, MD, a resident in the Department of Surgery, University of California, Los Angeles.
Seven patients with presumed stage II disease were found to have metastatic disease at surgery. In five of these patients, the presence of at least two circulating tumor cells was detected, he added.
The investigators found that circulating tumor cell enumeration could be used to discriminate between stages II, III, and IV disease (P = .013) and between local/regional disease and metastatic disease (P < .001). At a cutoff of at least two circulating tumor cells per 2 mL of blood, sensitivity was 68.8%, specificity was 96%, and positive predictive value was 92.3%. Circulating tumor cells were more discriminatory than CA 19-9 levels, Dr. Ankeny added.
“[Circulating tumor cells] show promise as a biomarker at the time of disease presentation that could be used for pretreatment staging,” he suggested.
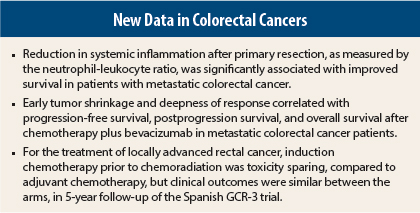
Systemic Inflammation and Colorectal Cancer Outcomes
Immune response impacts metastatic colorectal cancer tumor biology. Local inflammation with high-density tumor infiltrating lymphocytes is associated with good prognosis, while systemic inflammation, assessed by a neutrophil-lymphocyte ratio > 5, is associated with a poor prognosis. It is believed that a reduction in tumor burden (such as through surgical resection) might, in turn, reduce the level of tumor-promoting cytokines (ie, interleukin-6) that lead to systemic inflammation; in particular, reversal of a neutrophil-lymphocyte ratio > 5 (to ≤ 5) might improve patient survival.
Australian investigators explored this concept in 156 patients after primary resection for de novo metastatic colorectal cancer. This group’s median overall survival was 18.3 months.3 While there was a trend toward worse survival for those with a neutrophil-lymphocyte ratio > 5 at diagnosis (HR = 1.3; P = .15), postoperative neutrophil-lymphocyte ratio was a more impressive marker.
Patients with a postoperative neutrophil-lymphocyte ratio > 5 had a statistically significantly worse survival (HR = 2.37; P < .001) than those with a neutrophil-lymphocyte ratio ≤ 5, Primary resection resulted in reversal of neutrophil-lymphocyte ratio > 5 in 56% of patients, and this conveyed a significant survival advantage over persistence of neutrophil-lymphocyte ratio > 5 (HR = 0.53; P = .012). In fact, survival for patients with reversal of neutrophil-lymphocyte ratio > 5 was similar to those who had neutrophil-lymphocyte ratio ≤ 5 preoperatively, reported Philip V. Tran, MD, Director of Medical Imaging at the Western Hospital in Footscray, Australia.
Patients with the reversal of this potential biomarker of inflammation tended to have larger primary tumors but low metastatic burden (based on maximal diameter of largest metastasis).
Early Tumor Shrinkage and Deep Response
The importance of achieving an early response to treatment for metastatic colorectal cancer was confirmed in an Italian study in which early tumor shrinkage predicted improved progression-free survival, postprogression survival, and overall survival after chemotherapy plus bevacizumab (Avastin).4 Deepness of response also correlated significantly with these outcomes, reported Chiara Cremolini, MD, a medical oncologist at the Santa Chiara Hospital in Pisa, Italy.
Early tumor shrinkage was defined as the relative change in the sum of the longest diameters of RECIST target lesions at week 8, compared to baseline, with a cutoff value of 20%. Deepness of response was the relative change in the sum of the longest diameters of RECIST target lesions at the nadir, in the absence of new lesions or progression of nontarget lesions, compared to baseline.
The data came from the TRIBE study, which randomized 508 patients to FOLFIRI (leucovorin, fluorouracil [5-FU], irinotecan) plus bevacizumab or FOLFOXIRI (leucovorin, 5-FU, oxaliplatin, irinotecan) plus bevacizumab. Median progression-free survival was significantly improved with FOLFOXIRI/bevacizumab (P = .003), and significantly more of these patients achieved an early response (64% vs 51%, P = .006), and a higher extent of deepness of response (P = .0009) compared to the FOLFIRI/bevacizumab arm.
With the arms combined, both early tumor shrinkage and deepness of response predicted progression-free survival, postprogression survival, and overall survival.
Median progression-free survival was 12.7 months in patients achieving early tumor shrinkage and 10 months in those without (HR = 0.66, P < .0001). Median postprogression survival was 17.1 vs 10.7 months, respectively (HR = 0.64, P = .0005). Median overall survival was 35.8 vs 22.4 months, respectively (HR = 0.54, P < .0001), Dr. Cremolini reported.
Deepness of response greater than the median was associated with a median overall survival of 36.8 months, vs 21.3 months without (HR = 0.47; P < .0001). Moreover, for the first time, a significant correlation of the deepness of response with progression-free survival was demonstrated (HR = 0.61 [0.49–0.73], P < .0001).
“Both [early tumor shrinkage] and deepness of response correlate with progression-free survival, postprogression survival, and overall survival, irrespective of the treatment arm,” Dr. Cremolini said. “These data confirm the importance of achieving an early response in metastatic colorectal cancer and the value of both early tumor shrinkage and deepness of response as determinants of long-term outcomes and potential endpoints for clinical trials.”
Induction vs Adjuvant Chemotherapy in Rectal Cancer
The randomized phase II Spanish GCR-3 trial, involving 108 patients, evaluated the efficacy and toxicity of two strategies in locally advanced rectal cancer. Arm A received chemotherapy plus radiotherapy, followed by surgery and four more cycles of postoperative adjuvant CAPOX (capecitabine and oxaliplatin), and arm B received CAPOX followed by chemoradiation and surgery.
The study previously showed that the induction approach (arm B) allowed most patients to receive planned treatment, with less toxicity, and no compromise in pathologic complete response and complete resection rates. Investigators reported an updated analysis at a median follow-up of 5 years.5
At 5 years, disease-free survival was 64.3% in arm A and 62.1% in arm B (P = .85) and overall survival was 77.9% and 74.7%, respectively (P = .64). Local recurrences after macroscopically complete resection of the primary tumor occurred in 2.1% of patients randomly assigned to arm A and in 1.9% of patients randomly assigned to induction chemotherapy. The cumulative incidence of distant metastases at 5 years in the intention-to-treat population was 21.1% in the adjuvant arm and 23.2% in the induction arm.
“The induction approach, however, achieved a better toxicity profile and allowed for greater exposure to systemic therapy, without affect compliance to chemoradiation,” reported Carlos
Fernandez-Martos, MD, a medical oncologist at Fundacion Instituto Valenciano de Oncologia in Spain. ■
Disclosure: Dr. Goldstein is a consultant or advisor for and has received research funding from Celgene. Drs. Ankeny, Tran, Cremolini, and Fernandez-Martos reported no potential conflicts of interest. For full disclosures from the study authors, visit meetinglibrary.asco.org.
References
1. Goldstein D, El-Maraghi RH, Hammel P, et al: Updated survival from a randomized phase III trial (MPACT) of nab-paclitaxel plus gemcitabine versus gemcitabine alone for patients with metastatic adenocarcinoma of the pancreas. 2014 Gastrointestinal Cancers Symposium. Abstract 178. Presented January 17, 2014.
2. Ankeny JS, Hou S, Lin M, et al: Pancreatic circulating tumor cells as a diagnostic adjunct in pancreatic cancer. 2014 Gastrointestinal Cancers Symposium. Abstract 175. Presented January 17, 2014.
3. Tran PV, Sinnathamby M, Wong H-L, et al: Are the survival benefits associated with primary resection in de novo metastatic colorectal cancer mediated by reversal of systemic inflammation? 2014 Gastrointestinal Cancers Symposium. Abstract 464. Presented January 18, 2014.
4. Cremolini C, Loupakis F, Lonardi S, et al: Early tumor shrinkage and deepness of response to predict progression-free, postprogression and overall survival: Results from the phase III TRIBE trial. 2014 Gastrointestinal Cancers Symposium. Abstract 521. Presented January 18, 2014.
5. Fernandez-Martos C, Pericay C, Aparicio J, et al: Chemoradiation (CRT) followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant CRT and surgery for locally advanced rectal cancer: Results of the Spanish GCR-3 randomized phase II trial after a median follow-up of 5 years. 2014 Gastrointestinal Cancers Symposium. Abstract 383. Presented January 18, 2014.