The somatostatin analog lanreotide (Somatuline) depot extended the time to disease progression in patients with pancreatic neuroendocrine tumors, in a planned subgroup analysis of the CLARINET trial, Alexandria T. Phan, MD, of The Houston Methodist Hospital in Texas, reported at the 2015 Gastrointestinal Cancers Symposium.1
“Treatment options in advanced pancreatic neuroendocrine tumors are limited, and the optimal front-line therapy has not been defined by existing prospective studies,” Dr. Phan indicated.
The CLARINET trial reinforces earlier findings that suggest a somatostatin analog can have antiproliferative activity. The PROMID trial established an antiproliferative benefit for octreotide, but this drug is currently approved for symptom management in functional neuroendocrine tumors (ie, symptom relief) and not as an anticancer agent. PROMID included only patients with midgut, primarily grade 1, tumors and hepatic volumes of mostly 0% to 10%. CLARINET included patients with gastroenteropancreatic neuroendocrine tumors–neuroendocrine tumors (midgut, hindgut, pancreas) with grade 1 and 2 tumors, more than one-third of whom had hepatic volumes greater than 25%.
“These findings support lanreotide as the first and only somatostatin analog approved by the U.S. Food and Drug Administration (FDA) as an antitumor treatment to improve progression-free survival in patients with unresectable, well- or moderately differentiated, unresectable locally advanced/metastatic gastroenteropancreatic neuroendocrine tumors,” Dr. Phan said.
CLARINET Details
CLARINET is a phase III multicenter, randomized, double-blind, placebo-controlled 96-week randomized trial in which 204 patients received lanreotide depot 120 mg or placebo, by deep subcutaneous injection, every 28 days. Treatment with lanreotide significantly prolonged progression-free survival (hazard ratio [HR] = 0.47, P < .001); median progression-free survival was not reached in the lanreotide arm and was 18.0 months in the placebo arm.2 Based on its findings, on December 15, 2014, lanreotide was approved by the FDA.
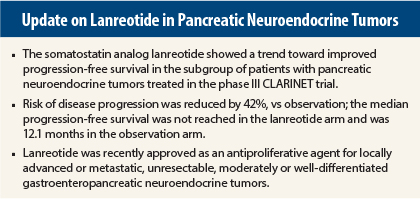
Subgroup analyses were done to investigate the consistency of treatment effects. The pancreatic neuroendocrine tumors subgroup included 91 patients with nonfunctional grade 1 or 2 tumors, representing about half of the broader CLARINET population. In this subgroup, 37% had hepatic tumor loads greater than 25%, 95% had stable disease at baseline, 38% had undergone surgery, and 77% had received no previous medical treatment.
The median progression-free survival was not reached in patients with pancreatic neuroendocrine tumors treated with lanreotide but was 12.1 months in the placebo arm (95% confidence interval [CI]: 9.4–18.3). This yielded a HR for progressive disease or death of 0.58 (95% CI: 0.32–1.04), which was not statistically significant but indicated a “favorable trend,” Dr. Phan said.
There were 18 events in the lanreotide arm, compared with 31 events in the placebo arm. In the lanreotide arm, 16 patients completed treatment without events, compared with 9 in the placebo arm. “The lanreotide treatment arm had more patients with disease control,” she said.
Although the mean exposure time was longer for lanreotide—62 weeks vs 56 weeks for the placebo arm—the incidence of adverse events (any events plus the most common events) was similar across the treatment arms. Adverse events were reported by 88% in each arm, and serious adverse events were reported by 29% in the lanreotide arm and 43% in the placebo arm (only three were treatment-related). The most common side effect was diarrhea, reported in 43% and 37%, respectively.
Consistent Across Age Groups
Dr. Phan also presented a prespecified exploratory analysis based on age, showing a consistent treatment effect of lanreotide 120 mg across age groups, with similar tolerability and safety.3 “These data offer reassurance that age is not a clinically significant factor when considering lanreotide for the treatment of pancreatic neuroendocrine tumors,” Dr. Phan said. “Both younger and older groups achieved antitumor effects, without tolerability issues.”
In the analysis of 115 patients younger than 65 and 89 patients aged 65 or older, the median progression-free survival was not reached with lanreotide in either age group and was 18.1 months in patients up to age 65 (HR = 0.51) and 12.1 months in those older than age 65 (HR = 0.38). ■
Disclosure: The CLARINET study was supported by a research grant from Ipsen, and Dr. Phan was one of the principal investigators. She is on advisory boards for Novartis and Ipsen; speakers bureaus for Novartis, Genentech, Lilly, and Celgene; and has received other research funding to her institution from Incyte, Novartis, Lexicon, and GSK.
References
1. Phan AT, Caplin ME, Pavel ME, et al: Effects of lanreotide autogel/depot in pancreatic neuroendocrine tumors: A subgroup analysis from the CLARINET study. 2015 Gastrointestinal Cancers Symposium. Abstract 233. Presented January 16, 2015.
2. Caplin ME, Pavel M, Cwikla JB, et al: Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 371:224-233, 2014.
3. Phan AT, Caplin ME, Pavel ME, et al: Effects of lanreotide autogel/depot in patients with neuroendocrine tumors age 65 or younger versus older than age 65: Subgroup analyses from the CLARINET study. 2015 Gastrointestinal Cancers Symposium. Abstract 367.