Positive results with the use of belantamab mafodotin-blmf in the phase III DREAMM-7 study were presented during the ASCO Plenary Series: February 2024.1 In relapsed or refractory multiple myeloma, DREAMM-7 evaluated the use of the regimen belantamab mafodotin, bortezomib, and dexamethasone (BVd) vs daratumumab, bortezomib, and dexamethasone (DVd). The investigators reported a highly significant improvement in progression-free survival along with a strong trend toward better overall survival with BVd.
“BVd demonstrated a statistically significant and clinically meaningful progression-free survival benefit, with a median progression-free survival that was 23 months longer than with DVd,” said Maria-Victoria Mateos Manteca, MD, PhD, Associate Professor of Medicine at the University of Salamanca and Director of the Myeloma Program at the Hospital Universitario de Salamanca, Spain.

Maria-Victoria Mateos Manteca, MD, PhD
Belantamab mafodotin is an antibody-drug conjugate targeting B-cell maturation antigen (BCMA). Prior disappointing findings from the phase III confirmatory DREAMM-3 trial led to the voluntary withdrawal of belantamab mafodotin in November 2022. DREAMM-3 compared single-agent belantamab mafodotin with pomalidomide and dexamethasone and failed to show a significant improvement in progression-free survival.2
About DREAMM-7
DREAMM-7 enrolled 494 patients with multiple myeloma previously treated with at least one prior line of therapy; they were randomly assigned to receive BVd or DVd. Belantamab mafodotin was given at 2.5 mg/kg every 3 weeks, and daratumumab and dexamethasone were dosed according to standard protocol. For cycle 9 onward, both belantamab mafodotin and daratumumab were given as single agents every 3 weeks and 4 weeks, respectively. The primary endpoint was progression-free survival.
Baseline characteristics were similar between the arms. About half the patients had stage II disease, and half had received one prior regimen. Approximately 27% of each arm had high-risk cytogenetics, and 5% of the BVd arm and 10% of the DVd arm had extramedullary disease. One-third of patients had disease refractory to lenalidomide, and more than two-thirds had received a stem cell transplant.
Benefit With BVd Regimen
After a median follow-up of 28.2 months, multiple outcomes were significantly improved with BVd (Table 1). Median progression-free survival, the primary endpoint, was 36.6 months in the BVd arm vs 13.4 months in the DVd arm (hazard ratio [HR] = 0.41; P < .0001). The progression-free survival benefit consistently favored BVd vs DVd across prespecified subgroups, including patients with lenalidomide-refractory (HR = 0.37) or high-risk cytogenetic (HR = 0.36) myeloma, Dr. Mateos reported.
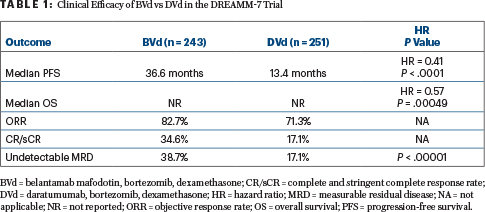
“Overall survival showed an early, strong, and clinically meaningful trend favoring the BVd arm as well,” she reported. Median overall survival was not reached in either arm, with events recorded for 22% of the BVd arm and 35% of the DVd arm (HR = 0.57; P = .00049). The threshold for statistical significance is P ≤ .00037. Follow-up for overall survival is ongoing.
BVd was also associated with a doubling in response rates and undetectable measurable residual disease. At 18 months, 76% of the BVd arm and 49% of the DVd arm were still responding to treatment.
Safety Profile
Half of the patients treated with BVd experienced serious adverse events compared with 37% of those treated with DVd; drug exposure was higher in the BVd arm. In general, Dr. Mateos noted, the safety and tolerability of the BVd regimen were consistent with the known safety profile of the individual agents.
Ocular adverse effects, which have been of concern with belantamab mafodotin, were observed in 79% of the BVd arm and 29% of the DVd arm, and they were grade ≥ 3 in 34% and 3%, respectively. Patients primarily reported blurred vision (66% overall, 22% grade ≥ 3), followed by dry eyes, eye irritation, and visual impairment (grade ≥ 3 in ≤ 7%). Ocular complaints led to dose reductions in 44%, dose interruptions in 78%, and treatment discontinuation in 9% of the BVd arm, but they were reported to be reversible in almost all patients. No difference was seen in global quality of life over time between the arms.
“These results suggest that BVd can potentially be a new standard of care in second-line or later relapsed and refractory multiple myeloma, owing to the robust efficacy, manageable safety, and ease of administration,” Dr. Mateos said. This recommendation applies irrespective of prior exposure to immunomodulatory drugs or protein inhibitors, she added.
DISCLOSURE: Dr. Mateos has served as a consultant or advisor to or has received honoraria from Janssen-Cilag, Celgene, Amgen, Takeda, GlaxoSmithKline, AbbVie/Genentech, Sanofi, Pfizer, Regeneron, Roche/Genentech, Stemline Therapeutics, and Kite/Gilead.
REFERENCES
1. Mateos MV, Robak P, Hus M, et al: Results from the randomized phase III DREAMM-7 study of belantamab mafodotin + bortezomib, and dexamethasone vs daratumumab, bortezomib, and dexamethasone in relapsed/refractory multiple myeloma. ASCO Plenary Series. Abstract 439572. Presented February 6, 2024.
2. Dimopoulos MA, Hungria VTM, Radinoff A, et al: Efficacy and safety of single-agent belantamab mafodotin versus pomalidomide plus low-dose dexamethasone in patients with relapsed or refractory multiple myeloma (DREAMM-3): A phase 3, open-label, randomised study. Lancet Haematol 10:e801-e812, 2023.

