Long-term follow-up of the phase III KEYNOTE-590 trial confirms the benefit of the PD-1 inhibitor pembrolizumab plus chemotherapy in advanced esophageal cancer. As compared with chemotherapy alone, after a median of follow-up of almost 59 months, patients treated with the chemoimmunotherapy combination were three times more likely to be alive at 5 years, with no appreciable increase in adverse events, according to Manish A. Shah, MD, FASCO, of Weill Cornell Medical College, New York, who reported these findings at the 2024 ASCO Gastrointestinal Cancers Symposium.1

Manish A. Shah, MD, FASCO
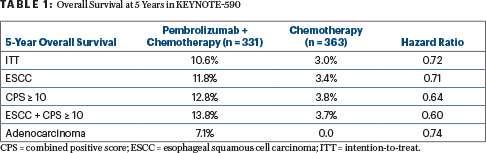
“There was a persistent and sustained difference in overall survival in the intention-to-treat population extending past 5 years,” said Dr. Shah, who reported 5-year overall survival rates of 10.6% with pembrolizumab plus chemotherapy and 3.0% with chemotherapy alone. “Achieving a 10% long-term survival rate is remarkable…. These data continue to support the use of pembrolizumab plus chemotherapy for advanced esophageal cancer as first-line therapy.”
About KEYNOTE-590
As previously reported, KEYNOTE-590 enrolled 749 patients with locally advanced or metastatic esophageal adenocarcinoma, esophageal squamous cell carcinoma (SCC), or gastroesophageal junction adenocarcinoma.2 Patients were randomly assigned to receive chemotherapy (5-FU and cisplatin), plus up to 35 cycles of pembrolizumab or placebo. The primary endpoints were overall survival and progression-free survival in the intention-to-treat population.
At the first interim analysis, after a median follow-up of 22.6 months, pembrolizumab plus chemotherapy significantly improved overall survival (hazard ratio [HR] = 0.73) and progression-free survival (HR = 0.65), establishing this approach as a first-line standard of care.
Updated Results
In the updated analysis, pembrolizumab plus chemotherapy led to a significant improvement in overall survival, with median overall survival of 12.3 months vs 9.8 months with chemotherapy alone (HR = 0.72). Significant benefits were also seen in the subsets of esophageal SCC (n = 548), a combined positive score (CPS) ≥ 10 (n = 383), and esophageal SCC plus a CPS ≥ 10 (n = 286; Table 1).
Median progression-free survival was 6.3 months with pembrolizumab plus chemotherapy vs 5.8 months with chemotherapyalone (HR = 0.64). Hazard ratios were 0.65 for the esophageal SCC subset, 0.51 for the CPS ≥10 subset, and 0.53 for patients with esophageal SCC and a CPS ≥10. No patient in the control arm was alive without disease progression after 3 years, whereas 8% of the experimental arm remained progression-free at that time. Quality-of-life outcomes were similar in the intention-to-treat population and prespecified subgroups.
Dr. Shah noted that the 3% of patients still alive at 5 years with chemotherapy alone had localized unresectable disease “and a great response to chemotherapy. As for the 10% of patients alive at 5 years in the chemoimmunotherapy arm, one-third had liver metastases, “which we were able to eradicate with this treatment,” he added. “We need to dive more into what happened to patients if they were rendered no evidence of disease.” The ongoing RENAISSANCE study is addressing the issue of whether eradication of limited metastatic disease improves outcomes, he said.
Expert Point of View
Session moderator Arathi Mohan, MD, Clinical Assistant Professor of Oncology at the Rogel Cancer Center, University of Michigan Health, Ann Arbor, commented on the KEYNOTE-590 update for The ASCO Post. “The combination of pembrolizumab plus chemotherapy in the front-line setting has shown benefit, but there are still questions about how to stratify for lower CPS [combined positive score],” she said. “Those data may be available, but they were not reported. We see that a lot in trials of immunotherapy/chemotherapy combinations; there is no further stratification in the lower-CPS subgroups. Those data need to be teased out to fully understand the treatment benefit.”
Dr. Mohan further commented on the new 5-year overall survival rates of around 11% to 14%, depending on the subset: “It’s hard for me as an oncologist to use the word ‘cure,’ but it’s pretty remarkable that some patients, even with liver metastasis, have disease control on this regimen.”
DISCLOSURE: Dr. Shah reported financial relationships with Astellas Pharma, Bristol Myers Squibb, and Oncolys BioPharma. Dr. Mohan reported no conflicts of interest.
REFERENCES
1. Shah MA, Sun JM, Shen L, et al: First-line pembrolizumab plus chemotherapy for advanced esophageal cancer: 5-year outcomes from the phase 3 KEYNOTE-590 study. 2024 ASCO Gastrointestinal Cancers Symposium. Abstract 250. Presented January 18, 2024.
2. Sun JM, Shen L, Shah MA, et al: Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): A randomised, placebo-controlled, phase 3 study. Lancet 398:759-771, 2021.

