Minimal residual disease after induction and consolidation for the treatment of acute leukemia might be eradicated by novel therapies, thus obviating the need for stem cell transplantation. That is the prediction of Matthew J. Wieduwilt, MD, PhD, Assistant Clinical Professor of Medicine at the University of California, San Diego, Moores Cancer Center in La Jolla, who described emerging treatments at the 2014 Highlights of ASH in North America meeting, held recently in Miami.
In multiple studies, the presence of minimal residual disease pre- and post-transplant has been associated with worse prognosis. Used as front-line agents, a number of compounds now in clinical trials may ameliorate this problem, he said. The most promising drugs are chimeric antigen receptor–modified T cells (CAR-T), inotuzumab ozogamicin, blinatumomab, and tyrosine kinase inhibitors.
Although still limited to small pilot studies in small numbers of patients, the findings for engineered T cells—so called CAR-T therapy—are impressive, and the excitement over this new class of agents was palpable at the American Society of Hematology (ASH) Annual Meeting. The cellular therapy involves isolating T cells from the patient and genetically modifying them with a chimeric antigen receptor construct. Expression of the CAR by a T-cell promotes binding of the CD19 antigen and triggers cytotoxicity, cytokine release, and T-cell proliferation, Dr. Wieduwilt explained.
CAR-T Therapy
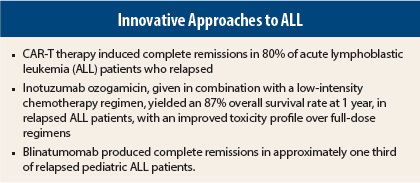
A phase I study of 16 patients (two in first remission [treated at relapse] and 14 relapsed at enrollment) was presented by Memorial Sloan Kettering investigators.1 Unfavorable cytogenetics were present in 44% of patients, and 25% were positive for the Philadelphia chromosome. After CAR-T cell infusion, more than 80% of patients achieved complete remission, compared to 52% who had this outcome after chemotherapy reinduction chemotherapy. In these groups, 70% were negative for minimal residual disease after CAR-T therapy, compared to only 10% after chemotherapy.
Approximately half the patients developed fevers, hypotension, hypoxia, neurologic changes, and/or malaise—ie, cytokine release syndrome—but this responded well to the anti–interleukin-6 antibody tocilizumab (Actemra).
Inotuzumab Ozogamicin
Also producing “impressive early results” were early studies of inotuzumab ozogamicin, a CD22 monoclonal antibody combined with a toxin conjugate that was used in combination with low-intensity chemotherapy in a small study of 17 patients ≥ 60 years old with newly diagnosed B-cell acute lymphoblastic leukemia (ALL).2
The patients received a low-intensity modification of the hyper-CVAD regimen referred to as mini-hyper-CVD (cyclophosphamide and dexamethasone at 50% dose reduction, no anthracycline, methotrextate at 75% dose reduction, cytarabine at 0.5 g/m2 for four doses), plus inotuzumab on day 3 of each of the first four courses.
Overall survival at 1 year was significantly improved over historical controls receiving full-dose hyper-CVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone): 87% vs 60% (P = .04). Of 14 patients evaluable for response, 13 (93%) achieved complete response or complete response with incomplete platelet recovery. All patients with complete response also achieved flow-cytometric minimnal residual disease–negative status. Additionally, the rate of deaths due to adverse events was 6%, considerably less than the 34% observed with hyper-CVAD.
Randomized front-line trials are now in development for younger adults, he said.
Blinatumomab
Results were a little less impressive with blinatumomab, a bispecific T-cell engaging antibody binding to CD3 and CD19, in relapsed pediatric acute lymphoblastic leukemia compared with CAR-T cells or inotuzumab ozogamicin, according to Dr. Wieduwilt. The drug acts independently of the T-cell receptor and allows T-cell recognition of tumor-associated surface antigen, without requiring major histocompatibility complex class I and/or peptide antigen.
In a study of 41 patients, the best response within two cycles was complete remission or complete remission with incomplete hematologic recovery in 37%, with 30% of those patients having no detectable minimal residual disease.3 Partial responses were observed in 7%. Randomized trials are in development in children and adults with ALL. ■
Disclosure: Dr. Wieduwilt reported consultancy for Cellerant and research funding from Sigma Tau.
References
1. Davilla M, Riviere I, Wang X, et al: Safe and effective re-induction of complete remissions in adults with relapsed B-ALL using 19-28z CAR DC19-targeted T cell therapy. 2013 ASH Annual Meeting. Abstract 69. Presented December 8, 2013.
2. Jain N, O’Brien S, Thomas D, et al: Inotuzumab ozogamicin in combination with low-intensity chemotherapy (mini-hyper-CVD) as frontline therapy for older patients (≥ 60 years) with acute lymphoblastic leukemia. 2013 ASH Annual Meeting. Abstract 1432. Presented December 7, 2013.
3. Von Stackelberg A, Zugmaier G, Handgretinger R, et al: A phase 1/2 study of blinatumomab in pediatric patients with relapsed/refractory B-cell precursor acute lymphoblastic leukemia. 2013 ASH Annual Meeting. Abstract 70. Presented December 8, 2013.