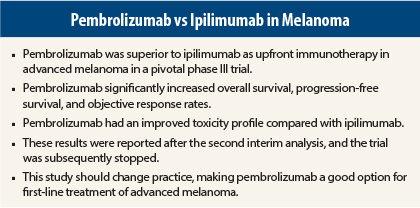
Pembrolizumab (Keytruda) proved superior to ipilimumab (Yervoy) for the treatment of unresectable advanced melanoma in the global phase III KEYNOTE-006 trial. Pembrolizumab significantly improved overall survival, progression-free survival, and overall response rate compared with ipilimumab, which is the current standard of care for the treatment of advanced melanoma. In addition, pembrolizumab was associated with fewer toxicities, including high-grade adverse events.
New Paradigm
KEYNOTE-006 was stopped early based on these promising results at the second interim analysis. Results were published in The New England Journal of Medicine1 to coincide with presentation of the data at the 2015 Annual Meeting of the American Association for Cancer Research (AACR).2
“The data from KEYNOTE-006 should change the standard of care for advanced melanoma regardless of whether patients have received prior ipilimumab therapy,” stated presenter and senior author of the study, Antoni Ribas, MD, PhD, Professor of Medicine and Hematology/Oncology and Director of Tumor Immunology at the Jonsson Comprehensive Cancer Center, University of California, Los Angeles.
“Not that long ago, we would be discussing treatments that helped 1 in 10 patients. With the development of agents that turn on the immune system, we now have therapies that help one in three patients. This is a new paradigm,” he stated.
Ipilimumab, an anti–cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) therapy, was the first drug of any kind to improve overall survival in patients with advanced melanoma, and it became the standard of care. Pembrolizumab is an anti–programmed cell death protein 1 (PD-1) antibody. Both are immune checkpoint inhibitors.
These immunotherapies have distinct mechanisms of action: blocking CTLA-4 enables T-cell activation, whereas blocking PD-1 modulates T-cell activity via the protein’s interaction with its ligands, PD-L1 and PD-L2, during the effector phase of the immune response. Two anti–PD-1 inhibitors, pembrolizumab and nivolumab (Opdivo), are approved by the U.S. Food and Drug Administration (FDA) for use in patients with disease progression during or after ipilimumab therapy, and if disease is BRAF V600 mutation–positive, after treatment with a BRAF inhibitor.
Study Details
KEYNOTE-006 included 834 patients from 83 sites in 16 different countries with unresectable stage III or IV advanced melanoma and no more than one prior systemic therapy. Patients were excluded if they received previous therapy with CTLA-4, PD-1, or PD-L1 inhibitors. Study participants were randomly assigned 1:1:1 to pembrolizumab at 10 mg/kg every 2 weeks (n = 279), pembrolizumab at 10 mg/kg every 3 weeks (n = 277), or four cycles of ipilimumab at 3 mg/kg every 3 weeks (n = 278).
Dr. Ribas explained that at the time KEYNOTE-006 was designed, the best dosing regimen of pembrolizumab was not known. The currently approved dosing regimen for pembrolizumab is 2 mg/kg every 3 weeks.
“There seems to be little difference between these dosing regimens [ie, all three pembrolizumab regimens],” he noted, “and there is no evidence that one of the two dosing regimens in KEYNOTE-006 is better.”
At a median follow-up of 7.9 months, median progression-free survival was 5.5 months for the every-2-week pembrolizumab group and 4.1 months for the every-3-week group, vs 2.8 months for ipilimumab, reflecting a 42% reduction in risk of disease progression with pembrolizumab (P < .00001).
Estimated 6-month progression-free survival rates were 47.3%, 46.4%, and 26.5%, respectively. “Progression-free survival is close to double with pembrolizumab,” Dr. Ribas noted.
At a median follow-up of 13.8 months, 1-year overall survival rates were 74.1% and 68.4% for the 2-week and 3-week pembrolizumab groups, respectively, vs 58.2% for ipilimumab, reflecting a 37% improvement in survival that was statistically significant (P = .00052 and P = .00358, respectively).
“This study exceeded our expectations, even for the control arm,” he said.
Objective response rates according to RECIST criteria for tumor shrinkage were about 33% in the pembrolizumab arms vs 11.9% in the ipilimumab arm. Complete response rates were 5%, 6.1%, and 1.4%, respectively. Responses were ongoing in 89.4% of the 2-week pembrolizumab group, 96.7% of the 3-week pembrolizumab group, and 87.9% of the ipilimumab group.
Improved Tolerability
“The toxicity profile favored pembrolizumab, with fewer side effects even though there was greater continuous exposure to the drug than with ipilimumab,” Dr. Ribas commented. Grade 3/4 toxicities were reported in 19% of the ipilimumab-treated patients and in 10% to 13% of those receiving pembrolizumab.
Treatment-related autoimmune- or immune-related adverse events with pembrolizumab were hypothyroidism (10.1% for the 2-week group and 8.7% for the 3-week group) and hyperthyroidism (6.5% and 2.5%, respectively). Colitis was reported in 8.2% of those assigned to ipilimumab.
“I’ve been treating melanoma for 15 years. This is the first time I have seen patients with durable responses, and the majority with no side effects. We saw no nausea, no vomiting, while the immune system is attacking the cancer. There is no better personalized therapy than this,” Dr. Ribas commented during the question-and-answer session following presentation of these data at an AACR press conference.
Dr. Ribas also said that pembrolizumab is approved for upfront use by Medicare and included as a first-line option in the recently released National Comprehensive Cancer Network Guidelines.
“The field is going faster than FDA approval. I anticipate that the FDA label [for pembrolizumab] will change to include first-line treatment. I won’t stop using ipilimumab, because this drug can achieve durable responses. It is part of our armamentarium. We highly anticipate that combining ipilimumab with an anti–PD-1 antibody will improve responses, but we need to know in which patients.” ■
Disclosure: Dr. Ribas has consulted for Merck, with the honoraria paid to his institution.
References
1. Robert C, Schachter J, Long GV, et al: Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. April 19, 2015 (early release online).
2. Ribas A, Schachter J, Long GV, et al: Phase III study of pembrolizumab (MK-3475) versus ipilimumab in patients with ipilimumab-naive advanced melanoma. AACR Annual Meeting 2015. Abstract CT101. Presented April 19, 2015.