
The reasons for low tolerability in the adjuvant setting may relate to there being less disease present and the agents’ not exerting an antiangiogenic effect on micrometastases. In theory, patients without metastatic disease may also be less tolerant of the side effects of these agents after curative surgery.— Cora N. Sternberg, MD, FACP
Tweet this quote
Renal cell carcinoma is the most common cancer of the kidneys. Up to 30% of patients present with advanced/metastatic disease, and recurrence can develop in patients at high risk treated by nephrectomy for localized tumors. Renal cell carcinoma is notoriously resistant to chemotherapy and hormonal therapy. Cytokine therapy with interferon-alfa and interleukin-2 (Proleukin) has been the backbone of treatment.
In the past decade, systemic treatment for patients with advanced and metastatic clear cell renal cell carcinoma (85% of cases) has undergone a dramatic shift, based on the knowledge that clear cell renal cancer is almost always associated with von Hippel–Lindau (VHL) mutation and dysregulated angiogenesis. Vascular endothelial growth factor (VEGF)-driven angiogenesis can be blocked by VEGF receptor tyrosine kinase inhibitors (VEGFR inhibitors) and also by inhibitors of the mammalian target of rapamycin (mTOR).
Sorafenib (Nexavar) and sunitinib (Sutent) were among the first antiangiogenic therapies to be approved in the advanced/metastatic setting for first- and second-line therapies, based primarily on improvement in progression-free survival. Since then, other agents have also been shown to improve progression-free survival and, more recently, also overall survival.
First Large Adjuvant Trial
Prior studies in the postoperative setting with chemotherapy, hormonal therapy, immunotherapy, and vaccine therapy have not been successful. As reported by Haas and colleagues in The Lancet and reviewed in this issue of The ASCO Post, ECOG-ACRIN E2805 (aka ASSURE) is the first large multi-institutional randomized double-blind trial (conducted at 226 centers in the United States and Canada) to report results of antiangiogenic therapies, successful in metastatic disease, in the adjuvant setting.1 Sorafenib, sunitinib, or placebo was given for 54 weeks in the postoperative setting to nonmetastatic patients at high risk for relapse after nephrectomy; 1,943 patients were enrolled.
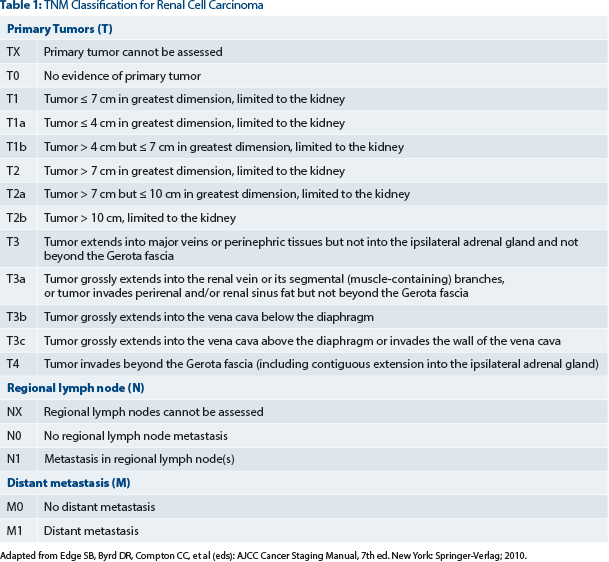
Patients had pathologic grade 3 or grade 4, pT1b to any higher T stage or grade, or node-positive (fully resected) disease (see Table 1). They were stratified by histology (clear cell vs non–clear cell), surgery (laparoscopic or open), Eastern Cooperative Oncology Group performance status, and UCLA risk category. The primary endpoint was disease-free survival. Secondary endpoints included overall survival, disease-free survival for clear cell disease, and safety.
The study was hampered by a high degree of grade 3 or higher side effects and dose reductions. Treatment was discontinued with sorafenib in 45% and sunitinib in 44% of patients. A reduction in the starting dose was necessary to improve compliance, and an amendment to expand accrual was instituted in 2009. The amended study was powered to test the original hypothesis of a 25% reduction in the hazard ratio, assuming a discontinuation rate of 23.4%, at a one-sided per comparison significance level of 1.25%.
Nonetheless, the percentage of patients with grade ≥ 3 side effects was still high (> 55%). Side effects consisted primarily of hypertension, hand-foot-syndrome, and fatigue. In the entire study, there were five deaths related to treatment or that occurred within 30 days after the end of treatment.
Low Tolerability in the Adjuvant Setting
This landmark study is the first of several large randomized adjuvant studies. Table 2 provides a summary of ongoing and completed adjuvant trials.

Unfortunately, other adjuvant studies, such as SORCE and PROTECT, have been hampered by similar problems with toxicity and the need to reduce the starting dosage of therapy due to compliance in the adjuvant setting. The reasons for low tolerability in the adjuvant setting may relate to there being less disease present and the agents’ not exerting an antiangiogenic effect on micrometastases. In theory, patients without metastatic disease may also be less tolerant of the side effects of these agents after curative surgery.
ECOG-ACRIN E2805 failed to reveal an improvement in disease-free survival; median disease-free survival was 5.8 years for sunitinib, 6.1 years for sorafenib, and 6.6 years for placebo. Median disease-free survival in the clear cell subset was 5.6 years for sunitinib, 5.6 years for sorafenib, and 6.6 years for placebo. Five-year overall survival was 77.9% for sunitinib, 80.5% for sorafenib, and 80.3% for placebo. The hazard ratio for both agents approached 1.0 compared to placebo for both disease-free survival and overall survival. This was true both in the original cohort and in patients who received a reduced initial dosage.
This first reported large adjuvant trial suggests that therapy with the VEGFR tyrosine kinase inhibitors sunitinib and sorafenib showed no benefit and a great deal of toxicity. Discontinuations and dose reductions occurred frequently. Further results of other ongoing trials are awaited, but we have curbed our enthusiasm in expecting highly positive results. ■
Disclosure: Dr. Sternberg has received honorarium and/or research support from Novartis, GlaxoSmithKline, Pfizer, Eisai, and Bayer.
Reference


