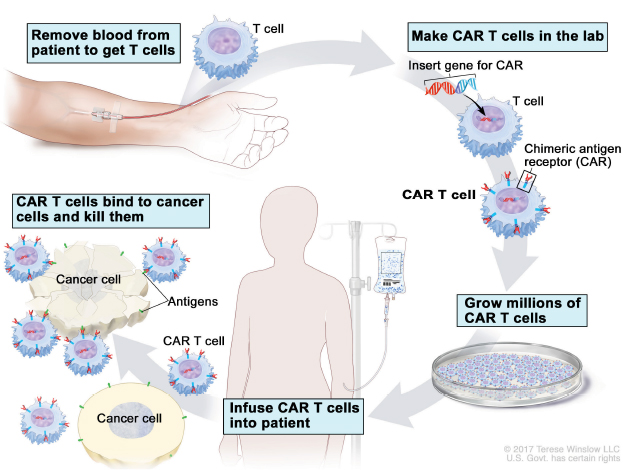
How It Works. The above image illustrates the process of making CAR T cells for each individual patient from collecting the patient’s T cells from their blood, shipping the cells to the laboratory for modification and manufacturing, to infusing the engineered CAR-containing T cells into the patient. © 2017 Terese Winslow LLC. U.S. Govt. has certain rights.
This past year’s approval by the U.S. Food and Drug Administration (FDA) of two chimeric antigen receptor (CAR) T-cell therapies heralded a new era in both effective cancer treatments and the most expensive cancer drugs ever. Tisagenlecleucel (Kymriah) was initially approved for the treatment of relapsed or refractory pediatric and young adult acute lymphoblastic leukemia (ALL; and has since been approved for adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy), and axicabtagene ciloleucel (Yescarta) was approved for the treatment of several types of relapsed or refractory large B-cell non-Hodgkin lymphomas (NHLs), including diffuse large B-cell lymphoma (DLBCL).
Additional analysis of data from the ELIANA clinical trial shows impressive results in 75 patients treated with tisagenlecleucel. The analysis found the overall remission rate within 3 months was 81%; the rates of event-free survival were 73% at 6 months and 50% at 12 months; overall survival was 90% and 76% over the same time intervals.1
Follow-up analysis of results from the ZUMA-1 trial investigating the effectiveness of axicabtagene ciloleucel in patients with refractory NHL also shows impressive outcomes. According to the analysis, more than 1 year after treatment, 42% of the 108 patients enrolled in the trial had maintained remission, and 40% of the patients exhibited no evidence of cancer. In addition, more than half of the patients were alive at the median follow-up of 15.4 months—more than double the median survival of 6.6 months for patients treated with conventional therapy.2,3
Sticker Shock
However, although these promising findings are generating much enthusiasm for CAR T-cell therapy—ASCO named adoptive-cell immunotherapy its 2018 Advance of the Year4—it is tempered by their hefty price tags: $475,000 for tisagenlecleucel and $373,000 for axicabtagene ciloleucel. And these prices reflect just the cost of extracting a patient’s T cells, engineering them to produce CARs on the surface of the cells, and infusing the cells back into the patient. They do not include fees for hospital stays, supportive care, or physician visits, which could drive the total cost to stratospheric levels of more than $1 million per patient, according to some reports.5
This past April, the Centers for Medicare and Medicaid (CMS) said it would reimburse hospitals about $400,000 for axicabtagene ciloleucel and $500,000 for tisagenlecleucel. Although outpatients usually have a 20% copayment on Medicare B services, which would mean a copayment of $79,076 for axicabtagene ciloleucel and $100,168 for tisagenlecleucel, the Social Security Act statute requires CMS to cap outpatient copayments at the amount of the inpatient deductible, which is $1,340 in 2018.
When tisagenlecleucel was approved in August 2017, Novartis made an outcomes-based agreement with the CMS, which calls for payment to the company for patients who show a morphologic remission within a month after receiving their CAR T-cell infusion. If the patient does not respond within the first month—a majority of patients, about 80%, have an initial response to the therapy—Novartis will not bill those medical facilities that opted in to the outcomes-based agreement.
Still, challenges associated with the pricing and reimbursement of CAR T-cell therapies remain and could become heightened as additional indications for these therapies are granted FDA approval and the population of eligible patients increases. For example, on May 1, 2018, the FDA expanded approval of tisagenlecleucel to the treatment of adult patients with relapsed or refractory large B-cell lymphoma, including DLBCL, high-grade B-cell lymphoma, and DLBCL arising from follicular lymphoma, after two or more lines of systemic therapy. And the manufacturer has announced plans to launch clinical trials in 2018 investigating tisagenlecleucel and other CAR T cells, in the treatment of multiple myeloma, DLBCL after first relapse, and follicular lymphoma, as well as solid tumors (including glioblastoma, advanced ovarian cancer, and mesothelioma).
Financial toxicity is not the only concern with this therapy. Both tisagenlecleucel and axicabtagene ciloleucel carry a black box warning for cytokine-release syndrome (CRS) and for neurologic events. To treat CAR T-cell–induced severe or life-threatening CRS, the FDA expanded the approval of tocilizumab (Actemra) and has required that CAR T-cell therapy be performed only in cancer centers that have completed training in Risk Evaluation and Mitigation Strategy to support its safe use. Currently, about 40 cancer centers are certified to offer CAR T-cell therapy to patients. (See sidebar on “Treatment Centers Authorized to Administer CAR T-Cell Therapy” on pages 49–50.)

Carl H. June, MD

Sagar Lonial, MD

David G. Maloney, MD

Pascal Touchon
To learn more about the clinical impact of CAR T-cell immunotherapies on patients with cancer; how oncologists, patients, and society should evaluate their cost vs value; and the future direction of cell immunotherapies in oncology care, The ASCO Post held a roundtable discussion with four leading experts in the field. They include Carl H. June, MD, Richard W. Vague Professor in Immunotherapy, Department of Pathology and Laboratory Medicine; Director, Center for Cellular Immunotherapies; and Director, Parker Institute for Cancer Immunotherapy, Perelman School of Medicine, University of Pennsylvania (in April, Dr. June was named one of TIME magazine’s 100 most influential people of 2018); Sagar Lonial, MD, Professor and Chair of Hematology and Medical Oncology; Chief Medical Officer, Winship Cancer Institute, Emory University School of Medicine; David G. Maloney, MD, PhD, Medical Director, Cellular Immunotherapy, Fred Hutchinson Cancer Research Center; Service Medical Director, Cellular Immunotherapy, Bezos Family Immunotherapy Clinic, Seattle Cancer Care Alliance; and Pascal Touchon, Senior Vice President and Global Head, Cell & Gene, Novartis Oncology.
Determining Clinical Benefit
Both tisagenlecleucel and axicabtagene ciloleucel are indicated for use in the relapsed setting as second- or third-line therapy. Should CAR T-cell therapy be reserved for the sickest patients, or do you see potential for its use as front-line therapy for those with high-risk disease?
Engineering T cells is a way to put the immune system on steroids and boost it to fight not just cancer but other chronic diseases as well.— Carl H. June, MD
Tweet this quote
Dr. June: Theoretically, like a lot of biologic therapies, it will be best to use cellular immunotherapies early in the course of a disease, but that will require randomized clinical trials to know for sure. There is a clinical study scheduled to begin this year testing CAR T cells upfront in high-risk patients with pre–B cell ALL, so we should have more information soon.
Dr. Lonial: I’m not a lymphoma expert, but with all the new therapies, when you are not certain as to where the risks and benefits are going to fall out, it is usually best to treat the sickest patients with the fewest options early on. There are subsets of patients with lymphoma who do quite well with standard chemotherapy, so the real question to me is can we identify the high-risk group of patients before they relapse and expose them to CAR T-cell therapy earlier in the course of disease, after first relapse. For instance, in the context of relapsed large B-cell lymphoma, is a CAR T-cell option better than an autologous transplant? There are clinical studies under way to answer these questions, but we’ll have to wait for the data to know for sure.
Dr. Maloney: Cell immunotherapies are not going to be immediately moved to the upfront setting, but the next studies in lymphoma will compare CAR T cells with standard of care for first-relapse disease, which is autologous stem cell transplant. To move CAR T-cell therapy to the actual front-line setting is going to require more work, at least in lymphoma, because the results are good with conventional chemotherapy for most patients. But as soon as conventional chemotherapy does not work in the front-line setting, I can certainly envision a time when CAR T cells would be second-line standard of care. We’re not quite there yet, but it will happen.
Mr. Touchon: We believe there is a place for this therapy in the earlier treatment setting, but we need to prove it is appropriate in specific clinical studies. We are planning a study this year with tisagenlecleucel in patients with DLBCL after first relapse and also a small trial of tisagenlecleucel in the first-line setting in very high–risk pediatric patients with B-cell ALL.
Clinical Experience
What has been your experience with patients receiving this therapy?
Dr. June: We have reported on the very high response rates and durable remission rates in patients with ALL. We have treated more than 300 patients with tisagenlecleucel in clinical trials, and a lot of that information was used for FDA approval of the therapy.
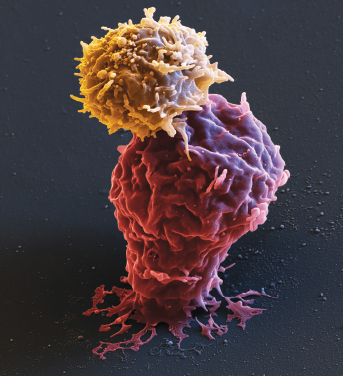
This illustration shows a color-enhanced scanning electron microscope image of a CAR T lymphocyte (beige color) attacking a leukemia cell (red color). The binding of the CAR T cell to the tumor cell initiates an immune defense against the cancer cell. Credit: Eye of Science/Science Source.
Dr. Lonial: In my experience treating patients with multiple myeloma, the benefit from this therapy has been quite striking. In a small clinical study of a second-generation CAR construct targeting the B-cell maturation antigen (BCMA) in multiple myeloma, the overall response rates looked even better than what we’ve seen with CD19 in lymphoma and ALL.6 Now, these are very, very early days, but I think the responses in durability are going to be quite impressive.
Dr. Maloney: We have also published extensively on our clinical trials data in chronic lymphocytic leukemia (CLL), lymphoma, and ALL, and there is clearly a high initial response rate with CAR T-cell therapy. We are seeing remission rates in patients in whom we did not see any type of remission with conventional therapies. Some patients relapse, and we don’t know whether patients are cured yet, and there can be serious toxicities with this therapy.
Retreatment After Relapse
Not all patients receiving CAR T-cell therapy respond to it or experience durable remissions. What happens to patients who relapse after this therapy? Can they be re-treated with CAR T-cell therapy?
Dr. June: To begin, there are CD19 CAR T cells, and the initial response rate and remission durations are very different depending on the type of cancer the patient has. The first patients we treated with CLL in 2010 are still in remission. We have the most durability data in patients with CLL, and it’s very rare to relapse, but the remission rate is about 50% currently. In ALL, the remission rate in multiple studies is between 80% and 90%. In one of our trials, there was a 28% relapse rate after 1 year, but after that, it is very rare to experience relapse, and the relapses that do occur are usually due to the loss of the CD19 part of the molecule on the leukemia cells. That’s different from what happens in lymphoma, where the complete response rate is 40%, and it’s rare to have relapses in those patients; when they do relapse, it is not usually with a CD19 target loss like in ALL. What happens after patients are treated depends on what type of tumor they have. There is not a “one-size-fits-all” treatment.
BEYOND CANCER: POTENTIAL INDICATIONS FOR CAR T-CELL THERAPY
- HIV/AIDS
- Hepatitis
- Lupus
- Arthritis
- Organ transplantation
- Inflammatory diseases
When patients relapse after conventional therapy, they may go on to other experimental therapies, including newer generations of CAR T cells. However, when patients relapse after CAR T-cell therapy, unfortunately, they usually die of their disease.
Dr. Lonial: Many trials are now allowing patients to be re-treated, so re-treating may make sense. What we’ve seen, particularly in lymphoma and ALL, is the idea that these patients were treated with very early-generation CAR T cells; if these were the only data we had, I probably would not be as excited about the potential for this therapy to revolutionize the field.
You’re right. Not all patients respond to the therapy, and the durability of responses can be limited; some of that may be due to patients dealing with end-stage disease, when it’s much more difficult to achieve a long-term response. For me, what shifted the potential applicability of CAR T cells in cancer were the data we saw in myeloma: Even in refractory disease, 90% of patients were having a major response to the therapy. And in the follow-up data we’ve seen, many of those patients, over two-thirds, are staying in remission close to a year out. These results suggested to me there may be modifications and evolution in the way we think about making CAR T cells and delivering them that may continue to improve outcomes for patients.
We are launching a trial at our center combining tisagenlecleucel with programmed cell death protein 1 (PD-1) inhibitors in patients with lymphoma to try to improve the longevity of the cell and ultimately the long-term antitumor effects of the therapy. It’s just a matter of figuring out, in a deliberate and scientific way, what are the best treatment combinations and who are the patients being treated; it is not just the CAR T-cell product we have to look at, it is the quality of the T cells being used to make the product as well. What we can do to the host to augment the production of effective T cells and what we can do to the cells once they have been extracted to augment their efficacy and long-term benefit are being investigated now.
Dr. Maloney: In my experience, it is seldom effective to re-treat patients with this therapy. Occasionally, the same patients may respond to the same CAR T cells, but in many cases, the reason they relapse and the reason the therapy may be ineffective are the patient’s immune system rejects the CAR T cells, because they are a foreign protein on the surface of the T cells. It’s important to note the three CARs likely to be approved in lymphoma in the near future, including JCAR017, all use the same murine single-chain Fv antibody—that means if a patient rejects one product, she or he is unlikely to respond to the other two.
However, a patient who rejects one CAR T cell may respond to a totally different CAR T cell. For example, a CAR T cell against a different target would be an option, but right now we don’t have very many other targets we are using in lymphoma. One option is CD22, so there is the potential of using a totally different CAR construct with a different antibody single-chain Fv, but we have to study this further.
Potential Mechanisms of Resistance
One theory as to why not all patients respond to this therapy is the T cells become so exhausted from repeated rounds of chemotherapy, they have decreased effector cytokine production and cytolytic activity. Another is the loss of the CD19 epitope may be a mechanism of resistance. Please talk about what may be driving resistance to this therapy.
Dr. June: Yes, that’s right. Some patients have been so badly beat up by chemotherapy, they may need someone else’s T cells to kill malignant cells. There is an emerging field now of allogeneic T cells, or “off-the-shelf” T cells, to solve the problem. But sometimes the tumor itself drives the resistance. The cells are like Houdini, and each one has its own escape mechanism. In ALL, it is by losing the target, and in lymphoma we think the tumor itself wears out the T cells. We need to find out what the issues are in each tumor and then design the T cells around those issues. And I think we have the scientific toolbox to do that.
Dr. Lonial: These two topics are the most important and relevant right now. First, you can have an insufficient effective T cell on the front end that you cannot make an effective effector cell, even if it is transformed, because of exhaustion or lack of function for any other reason. Second, I agree with Dr. June; the tumor cell figures out what it needs to survive and if the army coming after it is looking for CD19, it stops expressing CD19.
FDA-APPROVED CAR T-CELL THERAPIES
Tisagenlecleucel
- Approved August 2017, for treatment of relapsed or refractory acute lymphoblastic leukemia
- May 2018, expanded indication for treatment of relapsed or refractory diffuse large B-cell lymphoma
Axicabtagene ciloleucel
- Approved October 2017 for treatment of several types of relapsed or refractory large B-cell lymphoma non-Hodgkin lymphomas
This second problem is a lot more difficult to deal with and may be less of an issue if you treat patients earlier in their disease course, when there is less-resistant disease. Under study now is the issue of how much chemotherapy a patient has had and how effective the T cells are. One of the potential ways to solve the problem is to try to incubate the T cells with cytokines or other factors to activate them before they are infused into the patient. That may be a way to increase the efficacy of this therapy.
Dr. Maloney: It’s true that what may be contributing to the lack of sustained response in patients is their T cells are beat up because of prior therapy and they don’t have sustained cytokine production. Another reason may be the tumor microenvironment may be doing specific things that prevent the T cells from expanding, so adding checkpoint inhibitor antibodies may help. A third way patients may relapse, as Drs. June and Lonial explained, is the loss of the CD19 target. So, that’s immune escape, but it’s not a problem from the CAR T cells; it’s a problem from the tumor. The CAR T cells could not do any better than kill all the cells with the target. The only way to overcome the loss of CD19 is to target another antigen at the same time to prevent this kind of antigen-negative escape.
Mr. Touchon: I agree. One reason some patients with ALL do not respond to the therapy or relapse is because they become CD19-negative, and we are exploring dual-targeting CAR T cells to address this medical need. Another issue in DLBCL, as was mentioned, is that not all of a patient’s T cells may expand enough, and we are looking at ways to stimulate the expansion and activity of the cells in these patients, for example, with PD-1 blockers.
Potential in Solid Tumors
What is the potential of this therapy in solid tumor cancers?
Dr. June: There is more research now with this therapy in solid tumors, and we are beginning to see some responses, but this therapy is going to be more complicated in solid tumors than in bone marrow tumors. That’s the baseline answer, but I think we will eventually solve this problem.
Each cancer has its own Achilles heel, so using immunotherapy together with CAR T-cell therapy looks promising. Right now, the response rate with checkpoint therapy in solid tumors is about 20%, and I think when we combine it with engineered T cells, that percentage will increase, with the eventual goal of curing those patients.
Dr. Lonial: These are still very early days with this therapy. In blood cancers, we are fortunate the T cells tend to live in the blood, so they are always circulating through the bone marrow, which is our target organ. In solid tumors, the question is not just the target, but getting the T cells to be in that tissue and to stay there long enough to effect an antitumor response. What many groups, including investigators at our institution, are looking at is a different phenotype of T cell that is more tissue-resonate. The issue is how to identify those cells and make them effective T cells as opposed to a regular T cell that is floating in the blood. That’s what we’re trying to solve.
Dr. Maloney: The Holy Grail right now is trying to move the very encouraging results we have seen in the hematologic malignancies into the solid cancers. To date, the results in solid tumors have been disappointing. We have clinical trials in breast and lung cancers with CAR T-cell therapy under way now and are still learning how to get the dose right and understand the factors that make it difficult to treat these solid cancers.
Cost vs Value
A major concern of this therapy is its cost, which some estimates predict could go over $1 million per patient. Please talk about the cost vs value of CAR T-cell therapy.
SEE THESE VIDEOS FROM THE ASCO POST ON CAR T-CELL THERAPY
- Andrew D. Zelenetz, MD, PhD, and Sattva S. Neelapu, MD, on NHL: Results From the ZUMA-1 Trial
- Andrew D. Zelenetz, MD, PhD, and Stephen J. Schuster, MD, on DLBCL: Results of the JULIET Trial
- Bijal D. Shah, MD, on What ALL Tells Us About CAR T Cells
- Kristen Fousek, PhD Candidate, on B-Cell ALL: CAR T-Cell Treatment
- Stephen Gottschalk, MD, on CAR T Cells for Solid Tumors: What Are the Challenges?
Visit www.ascopost.com/videos to view these and other videos filmed by and for The ASCO Post.
Dr. June: Unfortunately, that is the cost of many existing noncurative cancer therapies when you sum up the cumulative costs of current therapies. There is competition now in the CAR T-cell therapy field, and the cost will come down eventually. Drug pricing of novel agents is a complex process, and it is more straightforward to set in different geographic regions. For example, in countries such as Canada and Japan, where there is a single-payer health-care system, drug prices can be more easily negotiated. The United States is probably the worst place in the world to open a new therapy because our system is so out of control. Competition will fix a lot of the pricing problem. If expensive therapies such as CAR T cells could be moved to the upfront setting and cured patients, $1 million would be a reasonable price to pay, and patients would be spared a lot of pain and suffering from enduring multiple noncurative therapies.
Dr. Lonial: I fully understand cost is a major concern for providers and patients, but my major focus is to eliminate myeloma in my patients. I agree we should have discussions about the cost of cancer therapy, but we should take a moment to realize how long it has taken us to get this kind of advance and appreciate what it means to the 30%, 40%, or 60% of patients who had no options at all and are now alive and well.
Dr. Maloney: This is a very challenging issue. You’re right that cost is a significant component of this therapy, and it is a major issue facing our health-care system. How are we going to pay for this therapy? And the cost is a challenge not just to patients and the health-care system, but to institutions as well.
In our center, we had to train over 400 people in the Risk Evaluation and Mitigation Strategy to be able to use one product, and that’s a huge burden on cancer centers. In addition, there are other logistical problems to overcome, including signing contracts with the drug manufacturers, and then you have to negotiate with insurance companies and CMS to cover the costs of the therapy. We need to come up with a better way to pay for these therapies.
Mr. Touchon: I agree that cost is an important topic. When we considered the price of tisagenlecleucel in the United States, the first thing we looked at was its medical value and the value it brings to patients, as evidenced by the ELIANA study. Then we looked at the value to the health-care system and society. Many of the patients with ALL treated with tisagenlecleucel were able to resume their normal life, and their parents and caregivers were also able to resume their normal lives, so the therapy has societal value in addition to patient value.
The health-care system value was based on the cost-effectiveness studies we conducted. The results from our studies found that pricing the therapy between $600,000 and $750,000 would have been cost-effective to the health-care system, but we decided instead to have a list price of $475,000. Since then, the National Institute for Health and Care Excellence and the Institute for Clinical and Economic Review have confirmed the cost-effectiveness of tisagenlecleucel.
It was important for Novartis to find a price for this one-time therapy that allows patients to access care through the U.S. health-care system and that also takes into account the innovation and investment to develop such a transformative therapy. We have implemented and support programs and outcomes-based agreements to further facilitate patient access to this therapy.
A Look Ahead
What is the future for CAR T-cell therapy in the treatment of cancer?
Dr. June: The future is not just in the treatment of cancer. Engineering T cells is a way to put the immune system on steroids and boost it to fight not just cancer but other chronic diseases as well, including human immunodeficiency virus (HIV)/AIDS and potentially hepatitis and autoimmune diseases such as lupus and arthritis. Companies are also investigating CAR T-cell therapy in organ transplantation to eliminate the need for lifelong immunosuppressants. The same principles that apply in the treatment of cancer apply to other diseases impacted by inflammation, and, eventually, this therapy will expand to these other diseases.
Dr. Lonial: In the future, we will bring this therapy into earlier and earlier stages of disease and in the process reduce the cost of treatment and the burden to patients. Figuring out who are the best candidates for CAR T-cell therapy and when such therapy should be given are questions currently undergoing research.
Dr. Maloney: The future is bright for CAR T-cell therapy. As we gain more experience with this therapy, the process will become safer, and more patients will benefit as we identify more targets and gain understanding of the biology of how CAR interacts with the T cells and what’s unique about each patient’s T cells that makes the therapy more or less effective.
It’s important to realize these are early days in this therapy, and even in cancers such as leukemia and lymphoma, in which these products are showing such stellar results, remission rates should get better and safer over time. Now, expanding the therapy to other cancers remains a challenge, but there will be an evolution of cellular-based immunotherapies, and we will learn how to successfully incorporate their use into other cancers.
Mr. Touchon: I agree. We are at the beginning of significant progress in transforming cancer care with cell immunotherapies, and it’s very exciting. We are developing other types of CAR constructs for targets in other hematologic cancers as well as in solid tumors, and we are investigating combining CARs with other agents to increase durable complete remission rates in as many patients as possible.
DISCLOSURE: Dr. June has sponsored research grants from Novartis, receives royalties from Novartis for intellectual property licensed by the University of Pennsylvania to Novartis, and is a scientific founder of Tmunity Therapeutics. Dr. Lonial reported no conflicts of interest. Dr. Maloney has received research funding from Juno Therapeutics and Kite Pharam received by Fred Hutchinson Cancer Research Center, and he is on the advisory boards of Kite Pharma, Novartis, and Eureka Therapeutics. Mr. Touchon is Senior Vice President and Global Head, Cell & Gene, Novartis Oncology.
REFERENCES
1. Maude SL, et al: Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 378:439-448, 2018.
2. Neelapu SS, et al: Long-term follow-up ZUMA-1. 2017 ASH Annual Meeting. Abstract 578. Presented December 11, 2017.
3. Neelapu SS, et al: Axicabtagene ciloleucel CAR-T cell therapy in refractory large B-cell lymphoma. N Engl J Med 377:2531-2544, 2017.
4. Heymach J, et al: Clinical Cancer Advances 2018. J Clin Oncol 36:1020-1044, 2018.
5. Szabo L: Cascade of Costs Could Push New Gene Therapy Above $1 Million Per Patient. Kaiser Health News. Available at https://khn.org/news/-cascade-of-costs-could-push-new-gene-therapy-above-1-million-per-patient. Accessed May 3, 2018.
6. Berdeja JG, et al: Durable clinical responses in heavily pretreated patients with relapsed/refractory multiple myeloma. 2017 ASH Annual Meeting. Abstract 740. Presented December 11, 2017.

