The KRAS mutation has long been considered “undruggable,” but new approaches in drug development may change this. The end result could be effective new treatment options for KRAS-mutated non–small cell lung cancer (NSCLC), according to David R. Gandara, MD, who described the emerging findings at the 15th Annual International Lung Cancer Congress in Huntington Beach, California.
“The KRAS mutation story has evolved from what we thought we knew, to the controversies about what we don’t know,” said Dr. Gandara, Professor of Medicine and Director of the Thoracic Oncology Program at the University of California, Davis, Comprehensive Cancer Center in Sacramento.
Key Concepts
Dr. Gandara described several key concepts regarding KRAS mutations:
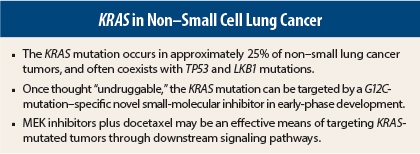
- KRAS mutations occur in approximately 25% of NSCLC tumors, compared to 39% of colorectal tumors. They are more likely to be transversions (78%) than transitions (22%), in contrast to colorectal cancer, where transitions are more common. This difference may explain the discordant response to treatment between the two tumor types.
- There are many different KRAS mutation subtypes, some but not all of which are associated with cigarette smoking.
- KRAS mutations in smokers may track together with other mutations, such as those in TP53 or Lkb1, which are tumor-suppressor genes.
- KRAS mutations—at least de novo mutations—are typically mutually exclusive of epidermal growth factor receptor (EGFR)-activating mutations and ALK translocations, though this is sometimes not the case, especially with ALK-positive tumors that have been exposed to an ALK inhibitor.
- There is an association between KRAS mutations and lack of response to EGFR tyrosine kinase inhibitors and chemotherapy, though the universality of this concept is now being questioned.
- A number of drug classes have targets downstream of KRAS, but no currently available drug inhibits KRAS directly.
Direct Targeting
A landmark paper published last year suggested it is possible to directly target a KRAS mutational subtype.1
“For the first time, we have data to suggest that we can target the KRAS G12C mutation with a small molecule,” he said. According to the study’s investigators, these compounds rely on mutant cysteine for binding, without affecting the wild-type KRAS protein. In other words, they are selective for a specific KRAS mutation subtype.
Cell growth inhibition and apoptosis were observed with KRAS targeting only in cell lines with the G12C mutation.
“The data suggest that these small-molecule inhibitors of KRAS are not only possible, but are quite effective in preclinical studies,” Dr. Gandara said. “They are directed toward the common G12C lung cancer mutation, which appears to be far more common in smokers than in nonsmokers, and they were developed to target only the mutated KRAS. In that regard they are very different from what we have had before.”
Targeting Downstream
Meanwhile, work continues on drugs that target downstream pathways and that indirectly affect KRAS. These efforts take advantage of the fact that KRAS mutations also have “traveling partners,” especially co-mutations in LKB1 (also known as STK11) and TP53, Dr. Gandara said.
Work from the The Cancer Genome Atlas (TCGA) has shown that the frequency of these co-mutations in NSCLC is similar to that of KRAS mutation alone. A very small percentage of patients have three simultaneous mutations.
It appears, from preclinical and clinical studies, that a MEK inhibitor plus docetaxel can effectively target these common co-mutations. In a preclinical study, mice were treated with docetaxel alone or with the investigational MEK inhibitor selumetinib.2 The animals harbored the KRAS G12D mutation or KRAS G12D mutation plus a mutation in either TP53 or LKB1, which are considered clinically relevant tumor suppressors.
In the study, the concomitant loss of either TP53 or LKB1 markedly impaired the response of KRAS-mutant cancers to docetaxel monotherapy. The addition of selumetinib provided substantial benefit for mice with lung cancer caused by KRAS and KRAS-plus-TP53 mutations, though mice with co-mutations in KRAS and LKB1 were resistant to the combination.
“There was a big difference in the waterfall plot among mice harboring these three categories of KRAS-mutated cancer. A rather dramatic change was seen with the MEK inhibitor in combination with docetaxel for all categories except LKB1 loss,” Dr. Gandara noted.
The phase II randomized human trial of selumetinib plus docetaxel in KRAS-mutant NSCLC was also positive for its primary endpoint, progression-free survival: 5.3 months with the combination vs 2.1 months with docetaxel alone.3 Response rates were 37% and 0%, and median overall survival times were 9.4 months and 5.3 months, respectively.
“Admittedly, this was totally KRAS-mutated NSCLC, which is not the typical patient, but the overall survival—though not statistically significant in this phase II study—was almost doubled with the combination,” he pointed out.
Trametinib Trials
Dr. Gandara presented a similar phase II study at the 2013 ASCO Annual Meeting for another MEK inhibitor, trametinib (Mekinist) plus docetaxel (with growth factor support) in advanced KRAS-mutant and wild-type KRAS NSCLC.4 Response rates were 28% to the combination among patients with KRAS-mutated NSCLC, 40% among those with G12C-mutated disease, and 32% among those with wild-type disease, and disease control rates were 60%, 80% and 68%, respectively.
“On the waterfall plot, we saw that all patients with G12C-mutated cancers had responses headed in the right direction, even if they did not meet RECIST criteria for a partial response,” he said.
While the response rates in the study were similar for patients with KRAS-mutated disease and those with wild-type disease, Dr. Gandara suggested the combination improved responses among those with mutated KRAS, compared to what would be expected with docetaxel alone. Both selumetinib and trametinib are moving forward in development for KRAS-mutated NSCLC, he indicated.
These data have led SWOG to initiate the randomized phase II S1408 trial in KRAS-mutated NSCLC, which will evaluate trametinib plus docetaxel or plus an AKT inhibitor (GSK795). The primary endpoint is progression-free survival.
Translational studies will evaluate outcomes in patients with the KRAS codon 12 (G12C) mutation vs other KRAS mutations, and for patients with TP53 mutations or LKB1 loss in each study arm. “This will, we hope, lead to subsequent phase III trials,” he added. ■
Disclosure: Dr. Gandara has received grants from GlaxoSmithKline and is a consultant for AstraZeneca and Novartis.
References
1. Ostrem JM, et al: K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature 503:548-551, 2013.
2. Chen Z, et al: A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response. Nature 483:613-617, 2012.
3. Jänne PA, et al: Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer. Lancet Oncol 14:38-47, 2013.
4. Gandara DR, et al: Oral MEK1/MEK2 inhibitor trametinib (GSK1120212) in combination with docetaxel in KRAS-mutant and wild-type advanced non-small cell lung cancer: A phase I/IIb trial. ASCO Annual Meeting. Abstract 8028. Presented June 2, 2013.