In a study that earned a Best Abstract Award at the 2019 Transplantation & Cellular Therapy (TCT) Meetings in Houston, minimal residual disease (MRD) negativity at 1 year after autologous hematopoietic cell transplant (HCT) and maintenance lenalidomide therapy was an independent prognostic factor for progression-free and overall survival in patients with multiple myeloma undergoing HCT.1 The TCT Meetings are cosponsored by the American Society for Transplantation and Cellular Therapy and the Center for International Blood & Marrow Transplant Research.

We think that measuring minimal residual disease at 1 year offers the largest value in terms of long-term prognosis for both progression-free and overall survival.— Theresa Hahn, PhD
Tweet this quote
Theresa Hahn, PhD, of Roswell Park Comprehensive Cancer Center, Buffalo, jointly led the multicenter study conducted by Roswell Park, the Center for International Blood and Marrow Transplant Research, the Medical College of Wisconsin, and the Blood and Marrow Transplant Clinical Trials Network. Marcelo Pasquini, MD, of Medical College of Wisconsin, co-led the study with Dr. Hahn. Dr. Hahn reported the results of the PRognostic Immunophenotyping in Myeloma Response (PRIMeR) study, which was the first major effort in the United States to examine MRD as an indicator of prognosis in this patient population. One thing that sets this study apart from previous studies, according to Dr. Hahn, is that it measured MRD by next-generation flow cytometry at three key junctures of treatment: before transplant (baseline), after transplant but before maintenance therapy is initiated (premaintenance), and 1 year after HCT (1 year).

Marcello Pasquini, MD
PRIMeR was an ancillary study of the phase III STaMINA trial (BMT CTN 0702, ClinicalTrials.gov identifier NCT01109004), which evaluated single autologous HCT vs tandem autologous HCT, vs single autologous HCT followed by four cycles of RVD (lenalidomide, bortezomib, dexamethasone).2 The STaMINA trial revealed no significant difference between these three treatment strategies for progression-free or overall survival. Patients in the STaMINA trial could opt in to participate in PRIMeR, the final data set of which included 437 patients, who contributed 883 bone marrow samples for MRD analysis.
Patients were evaluated by a method that is now considered next-generation flow cytometry. It included a panel of 11 different monoclonal antibodies and provided a sensitivity of 0.004% to 0.001%, detecting MRD in up to 1 million “events” analyzed per sample. “This is a high-level, very sensitive test. It’s on the same level as a molecular next-generation sequencing or polymerase chain reaction assay,” Dr. Hahn noted. This flow cytometry test mirrors what is now done in clinical practice.
Postmaintenance MRD Predictive
The rates of MRD negativity between the 3 treatment arms did not significantly differ among the baseline samples (40%–47%) and the premaintenance samples (76%–83%); however, at the 1-year time point, MRD negativity was higher in the tandem HCT group (92%) vs the autologous HCT/RVD group (85%) and autologous HCT group (78%). The P value for the 3-way comparison was .04.
Dr. Hahn said this does not suggest that tandem HCT is better than single HCT or autologous HCT/RVD, since these numbers are for the intent-to-treat population. “Many patients in the tandem-transplant group received a single transplant,” she explained. By actual treatment received, the MRD negativity rate did not differ: 81% for the autologous HCT group, 85% for the autologous HCT/RVD group, and 90% for the tandem-HCT group (P = .3).
MRD by Disease Status Before Transplant
By disease status before transplant, the numbers show that as the depth of response increases, so does MRD negativity, as has previously been reported. The rates of MRD negativity were 96% for patients in stringent complete response, 67% for those with a complete response, 59% for those with a very good partial response, and 22% for those with a partial response.
“Some studies have looked at MRD only in complete responders; however, even in patients with just a partial response, you see that a quarter are MRD-negative,” Dr. Hahn said. For patients achieving a very good partial response or better, 67% were MRD-negative, but patients with less than a very good partial response still had an MRD-negative rate of 23%. “This shows the value of measuring MRD regardless of response,” she emphasized.
Nuances Observed
Some patients, in fact, can achieve a complete response but be MRD-positive, whereas patients who achieve less than a complete response can be MRD-negative. Still, complete responders who are also MRD-negative fare better than complete responders who are MRD-positive. In discrepant groups, she said, “something else is going on…. MRD status is picking up something unique about these patients that adds to the depth of traditional response criteria. It can help further stratify patients within and across response categories.”
Conversion rates after 6 to 9 months of maintenance therapy were similar among the 3 treatment groups, with 30% of patients in each group converting from MRD-positive to MRD-negative. “This finding supports the overall STaMINA results: no difference in progression-free or overall survival between the 3 treatment arms,” she said.
Progression-Free and Overall Survival
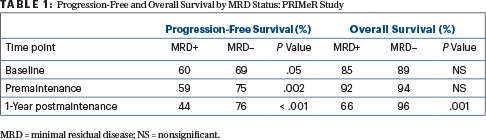
By MRD status, there was a significant improvement in progression-free survival for patients who were MRD-negative vs MRD-positive at all three time points measured. However, for overall survival, the 1-year time point alone was statistically significant, she reported (Table 1, page 25).
Minimal Residual Disease in Multiple Myeloma
- The PRIMeR study, which is a subanalysis of the STaMINA trial, is the first U.S. study of minimal residual disease (MRD) for multiple myeloma as part of a national randomized trial.
- A unique study design was used; it had three serial measurements of MRD, using next-generation flow cytometry.
- MRD negativity 1 year after transplant was prognostic for improved progression-free and overall survival.
- There was no difference in conversion from positive to negative MRD status according to treatment (single transplant, tandem transplant, or single transplant with consolidation therapy).
- MRD status offers independent prognostic information in patients with myeloma.
“Our study is the first to look at MRD at 1 year after autologous HCT and maintenance, and we show that the 1-year time point may be the most important for long-term prognosis,” Dr. Hahn said. “At 1 year, progression-free survival is very different by MRD status, but we also found the same large difference for overall survival. We think that measuring MRD at 1 year offers the largest value in terms of long-term prognosis for both progression-free and overall survival.”
Multivariable Analysis
In the STaMINA study, the only independent predictor for progression-free survival was high-risk disease, showing a hazard ratio of 1.66 compared with standard-risk disease. When MRD status at 1 year is added to this analysis, the hazard ratio for high-risk disease is 3.29, and for MRD is 4.37.
“Both high-risk disease and MRD status are significant independent predictors of progression-free and overall survival. Both variables are picking up something distinct about the patient’s myeloma biology,” she said.
Future Directions
The investigators will continue to follow patients and update the survival analysis with 6 years of follow-up data. “We will try to tease apart what looks like a higher MRD negativity rate in the tandem-transplant group by intent-to-treat vs actual treatment. We also want to compare patients who are MRD-negative at all time points with those who convert from positive to negative and try to understand more about complete responders who remain MRD-negative. We want to know what’s different about these patients,” Dr. Hahn said. ■
DISCLOSURE: Dr. Hahn has received an institutional research grant from Celgene.
REFERENCES
1. Hahn TE, Wallace PK, Fraser R, et al: Minimal residual disease (MRD) assessment before and after autologous hematopoietic cell transplantation (AutoHCT) and maintenance for multiple myeloma: Results of the Prognostic Immunophenotyping for Myeloma Response (PRIMeR) study. 2019 Transplantation & Cellular Therapy Meetings. Abstract 6. Presented February 22, 2019.
2. Stadtmauer EA, Pasquini MC, Blackwell B, et al: Autologous transplantation, consolidation, and maintenance therapy in multiple myeloma: Results of the BMT CTN 0702 trial. J Clin Oncol 37:589-597, 2019.


