In higher-risk myelodysplastic syndromes (MDS), a global phase II open-label study found that the combination of pevonedistat plus azacitidine was associated with a trend toward improved event-free survival and numerically longer overall survival, as compared with azacitidine alone, according to Lionel Ades, MD, PhD, of the Hôpital Saint-Louis, Paris.1
“Encouraging clinical efficacy with pevonedistat plus azacitidine was observed in higher-risk MDS and low-blast acute myeloid leukemia [AML]. In higher-risk MDS, the combination led to longer event-free survival, numerically longer overall survival, longer duration of response, and double the complete response rate, as compared with azacitidine alone,” he said. “In low-blast AML, overall survival trended longer with pevonedistat and azacitidine.”

Lionel Ades, MD, PhD
Need for New Approaches
Dr. Ades noted the need for novel therapies that can be combined with azacitidine for patients with higher-risk MDS, chronic myelomonocytic leukemia (CMML), or low-blast AML ineligible for high-intensity therapy or transplant. “Attempts to enhance azacitidine’s efficacy by adding other agents have been limited by premature discontinuation or dose adjustments due to increases in adverse events,” he added.
“Encouraging clinical efficacy with pevonedistat plus azacitidine was observed in higher-risk MDS and low-blast AML.”— Lionel Ades, MD, PhD
Tweet this quote
Treatment with pevonedistat, the first small-molecule inhibitor of the NEDD8-activating enzyme, disrupts cell-cycle progression and survival. In AML, the drug has shown encouraging activity and tolerability in combination with azacitidine, Dr. Ades said.
Study Details
The global phase II randomized, open-label study included 120 patients with higher-risk MDS (56%), low-blast AML (30%), and higher-risk CMML (14%) or who had not received a hypomethylating agent and were ineligible for allogeneic stem cell transplant. Patients were randomly assigned to pevonedistat at 20 mg/m2 on days 1, 3, and 5 plus azacitidine at 75 mg/m2 on days 1 to 5, 8, and 9 or azacitidine on the same schedule, repeated every 28 days. Study endpoints were event-free survival (time to death or transformation to AML in higher-risk MDS/CMML or death in low-blast AML), overall survival, and objective response rate.
“The study was powered for event-free survival as the original primary endpoint. Overall survival was a secondary endpoint but was changed to the primary endpoint based on regulatory feedback after enrollment,” noted Dr. Ades.
Trend for Longer Survival in Intent-to-Treat Population
In the intent-to-treat population, there was a trend toward longer event-free survival with pevonedistat/azacitidine compared with azacitidine alone: median 21.0 vs 16.6 months (hazard ratio [HR] = 0.665; P = .076). Median overall survival was 21.8 vs 19.0 months, respectively (HR = 0.802; P = .334), Dr. Ades reported.
“The study was powered to assess differences in event-free survival, not overall survival,” he noted. Median overall survival with azacitidine was consistent with the azacitidine control arms in other phase II studies.
Benefits in Higher-Risk Subset
The study was also not powered to assess differences in subgroups, but patients with higher-risk MDS had a significantly longer event-free survival with the combination vs azacitidine alone: median 20.2 vs 14.8 months (HR = 0.539; P = .045). Median overall survival in this subgroup was also numerically higher: 23.9 vs 19.1 months, respectively (HR = 0.701; P = .240; Table 1).
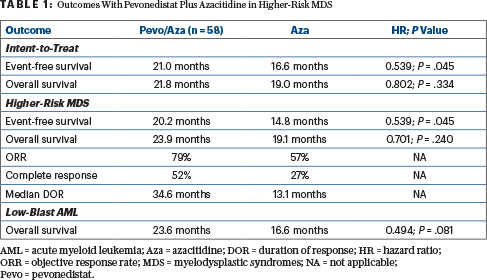
Analysis of patients with higher-risk MDS by prespecified subgroups showed the benefit of the combination in patients whose risk was deemed “very high” or “high” by the International Prognostic Scoring System Revised version. For these subsets as well as for patients with low-blast AML, the combination was effective in patients harboring poor-risk mutations, reported Dr. Ades.
Response rates in the higher-risk MDS subgroup were increased with the combination over azacitidine alone: 79% vs 57%, with complete responses seen in 52% and 27%, respectively. The median duration of response in these responders was 34.6 vs 13.1 months.
For patients with higher-risk MDS who were red blood cell transfusion–dependent at baseline, transfusion independence was achieved by 69.2% with the combination and 50.0% with azacitidine alone.
Low-Blast AML and CMML Subgroups
In patients with low-blast AML, there was a trend toward longer overall survival with the combination over azacitidine alone: 23.6 months vs 16.6 months (HR = 0.494; P = .081). In low-blast AML, by definition overall survival and event-free survival were equivalent.
KEY POINTS
- A global phase II study evaluated pevonedistat plus azacitidine vs azacitidine alone in patients with higher-risk MDS, CMML, and low-blast AML who are ineligible for high-intensity therapy or transplant.
- Pevonedistat plus azacitidine was associated with a trend toward improved event-free survival and numerically longer overall survival, as compared with azacitidine alone, in the intent-to-treat analysis.
- In the higher-risk MDS subset, a significantly longer event-free survival was achieved with the combination: median 20.2 vs 14.8 months (HR = 0.539; P = .045).
In the CMML subgroup, pevonedistat/azacitidine did not appear to improve efficacy over azacitidine alone, but the size of this subgroup precluded meaningful conclusions, according to Dr. Ades. In these smaller subsets of low-blast AML and higher-risk CMML, there was less of a difference in response rates.
Quality of Life and Safety
No differences between treatment arms were found in terms of quality of life. “Similar scores were maintained from study entry to the end of treatment,” Dr. Ades added.
Pevonedistat/azacitidine had a comparable safety profile to azacitidine alone; the dose intensity of azacitidine in the combination regimen was maintained. The median number of azacitidine treatment cycles was 13 with the combination and 8.5 as monotherapy.
DISCLOSURE: Dr. Ades has served as a consultant or advisor to and received research funding from Takeda, Celgene, and AbbVie.
REFERENCE
1. Ades L, Radinoff A, Sangerman MA, et al: Phase 2 study of pevonedistat + azacitidine versus azacitidine in patients with higher-risk myelodysplastic syndromes/chronic myelomonocytic leukemia or low-blast acute myelogenous leukemia. EHA25 Virtual 2020 Congress. Abstract S182.

