For the prevention of chemotherapy-induced nausea and vomiting, NEPA, a novel combination of a neurokinin-1 (NK1) receptor antagonist and the 5-HT3 receptor antagonist palonosetron (Aloxi), has been studied in three pivotal trials that were recently published in the Annals of Oncology.1-3 Further analyses of these data were presented at the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) International Symposium on Supportive Care in Cancer.
NEPA is an oral fixed-dose combination containing 300 mg of netupitant (a highly selective NK1 receptor antagonist) and 0.50 mg of palonosetron (a pharmacologically and clinically distinct 5-HT3 receptor antagonist). The combination targets dual antiemetic pathways and thus provides more complete control than 5-HT3 receptor antagonists alone.
The single-capsule oral administration of the combination may improve adherence to the guidelines for prevention of chemotherapy-induced nausea and vomiting, according to Matti S. Aapro, MD, of the Multidisciplinary Oncology Institute, Clinique de Genolier, Switzerland.
“The development of NEPA is an opportunity to overcome some barriers interfering with guideline adherence by targeting two critical pathways involved in emesis with a single oral dose,” Dr. Aapro said. “It further simplifies treatment because of the long effect of palonosetron, which allows us to not use corticosteroids on days 2 and 3.”
Key Findings
At the MASCC/ISOO meeting, held recently in Miami Beach, Dr. Aapro and other investigators reported that NEPA is effective across multiple chemotherapy cycles, controls not only emesis but also nausea, is protective in patients receiving carboplatin-based chemotherapy (for whom guidelines are not clear), and is not affected by cisplatin’s chemotherapy partner.
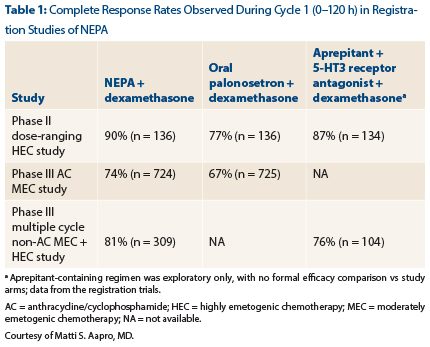
Dr. Aapro reported the key results from the registration studies in chemotherapy-naive patients (Table 1). The primary endpoint was complete response, which was defined as no emesis and no use of rescue medications.
In the phase II HEC [highly emetogenic chemotherapy] study and the phase III AC [anthracycline/cyclophosphamide] MEC [moderately emetogenic chemotherapy] study, the difference between NEPA/dexamethasone and oral palonosetron/dexamethasone was statistically significant (P < .01).
Study Details
The phase III, multinational, randomized, double-blind study enrolled 1,455 patients, of whom 1,286 participated in the multiple-cycle extension of the study. NEPA was given to 635 patients, while 651 were treated with oral palonosetron, for a total of 5,969 total chemotherapy cycles.
Dr. Aapro reported that NEPA exerted continued benefit throughout several cycles of chemotherapy. “NEPA showed superior 0- to 120-hour complete response rates compared with oral palonosetron over all cycles. All the differences were statistically significant.… We saw very encouraging persistence of the protection for patients overall,” he said.
At cycle 4, no emesis was reported by 87% of the NEPA arm vs 79% of the control arm (P = .0003). In addition, no significant nausea was reported by 80% and 75%, respectively (P = .042). No significant nausea was defined as maximum < 25 mm on the 100-mm visual analog scale.
“Treatment-related adverse events were quite rare (4% at cycle 4), with no differences between the arms and no indication of an increase in neutropenia. This is very important, as many patients and physicians ask about potential interactions of NEPA with chemotherapy,” he added.
“We believe a single fixed-dose of NEPA plus dexamethasone on day 1 of chemotherapy offers the potential for guideline-based prophylaxis with a convenient single-dose treatment,” Dr. Aapro concluded.
Nausea Control
“We have made tremendous progress in [chemotherapy-induced nausea and vomiting] prevention over 30 years, yet challenges remain. Nausea control, especially, is clearly suboptimal,” said Lee S. Schwartzberg, MD, FACP, of the West Clinic, Memphis.
Debate continues as to whether NK1 receptor antagonists enhance nausea control. The addition of these agents to 5-HT3 receptor antagonists plus dexamethasone has not shown consistent superiority over 5-HT3 receptor antagonists alone in terms of nausea control; however, the combination has clearly proved its worth in controlling emesis. The study presented at MASCC by Dr. Schwartzberg evaluated whether NEPA offers better control of nausea than single-agent oral palonosetron.
“We found that the addition of the NK1 receptor antagonist netupitant significantly improved the overall prevention of nausea over palonosetron alone in patients receiving cisplatin or AC,” he reported. “As expected, the absolute benefit was smaller for the prevention of nausea than it was for the prevention of vomiting.”
The analysis is from the multinational, randomized, double-blind registration trials of patients who received cisplatin-based highly emetogenic chemotherapy, which was study 1 (n = 271), and those who received moderately emetogenic chemotherapy with AC, which was study 2 (n = 1,449). Both compared NEPA to oral palonosetron; patients also received oral dexamethasone (12 mg with NEPA, 20 mg with palonesetron) on days 1 to 4 in study 1 and on day 1 in study 2.
In the primary analysis, rates of “no emesis” were significantly higher in the NEPA arms of both studies for the acute phase, delayed phase, and overall time period. For the current analysis, the proportion of patients reporting no significant nausea was significantly higher, as well, in the NEPA arms.
“With NEPA, we saw a statistically significant improvement in the proportion of patients who had nausea control overall and in the acute and delayed phases,” Dr. Schwartzberg reported.
About 90% of patients receiving NEPA in study 1 (highly emetogenic chemotherapy recipients) reported no significant nausea, compared to 79% with oral palonosetron (P = .021). The odds ratios favoring NEPA ranged from approximately 2.2 (delayed phase and overall) to 4.7 (acute phase).
Mean visual analog scale score (on a 100-mm scale) was also significantly lower in the NEPA arm for the acute, delayed, and overall analyses: 3.0, 7.5, and 8.0, respectively, compared to 6.5, 15.2, and 16.9 for oral palonosetron.
In study 2 (the AC moderately emetogenic chemotherapy population), no differences were observed in the acute phase, but NEPA provided a significant improvement in nausea control in the delayed phase (77% vs 71%, P = .014) and overall analysis (75% vs 69%, P = .020). The numerical improvement in percentage of patients with no significant nausea was somewhat less—approximately 7% overall—and mean visual analog scale scores, which were higher in study 2 than study 1, were numerically lower with NEPA.
“The beneficial effect on nausea was greater with cisplatin than for AC,” Dr. Schwartzberg pointed out.
On the Functional Living Index–Emesis questionnaire, significantly more patients on NEPA reported no impact on daily living for both the vomiting and nausea domains, he added.
Use With Carboplatin
Karin Jordan, MD, of the University of Halle, Germany, reported on the use of NEPA plus dexamethasone in patients receiving moderately emetogenic chemotherapy, noting that clinical guidelines for its use in this setting are not consistent.
“NK1 receptor antagonists are a standard of care in the treatment of patients with [highly emetogenic chemotherapy], but in the [moderately emetogenic chemotherapy] setting their use is not presently recommended by MASCC,” she indicated. “With ASCO, it’s kind of a ‘may consider’ situation. The National Comprehensive Cancer Network guidelines recommend them in selected patients.”
Dr. Jordan and colleagues performed a post hoc analysis from a prospective phase III trial of 413 patients to assess NEPA’s effectiveness in the subset of 140 patients receiving carboplatin. The endpoint during the overall phase (0–120 h) was the proportion of patients with no emesis.
“High rates of ‘no emesis’ were seen following a single dose of NEPA plus dexamethasone in the carboplatin subset, and the antiemetic efficacy was maintained over the cycles,” she reported.
The proportions of patients reporting no emesis were 83% in cycle 1, 91% in cycle 2, 92% in cycle 3, and 95% in cycle 4. The benefit with carboplatin was similar to that seen with cisplatin in other studies; it is more than 10% greater than the historical control rate of 70% and at least as good as what has been observed with the aprepitant three-drug regimen in both the cisplatin and carboplatin settings. Dr. Jordan cautioned, however, that while this is the largest prospective cohort study to evaluate the contribution of NK1 receptor antagonists in patients receiving carboplatin, it is a post hoc analysis and the data are descriptive only.
Nevertheless, she suggested, “Given the consistent evidence in almost 300 patients treated with NEPA/dexamethasone and previously with the aprepitant triplet, guidelines should consider the addition of an NK1 receptor antagonist in patients receiving carboplatin.”
Impact of Cisplatin’s Chemotherapy Partner
Paul J. Hesketh, MD, of Lahey Hospital & Medical Center, Burlington, Massachusetts, reported that NEPA was equally effective whether cisplatin was partnered with an agent of lower or higher emetogenic potential.
Cisplatin is the most emetogenic of the commonly used agents, and data suggest that whatever chemotherapy is added to cisplatin can affect antiemetic control. For example, the triplet of aprepitant, ondansetron, and dexamethasone proved less efficacious when anthracyclines and/or cyclophosphamide were added to cisplatin.
Dr. Hesketh, therefore, led a post hoc analysis of the phase II dose-ranging trial to further explore this question in 265 patients who received cisplatin plus an agent of lower emetogenic potential (most often fluorouracil, etoposide, or none) generally received by patients with lung, respiratory tract, and head and neck cancers, and 142 patients receiving cisplatin plus an agent of higher risk (cyclophosphamide, doxorubicin), typically ovarian and breast cancer patients.
The overall response rate was 89% for the lower-risk group and 87% for the higher-risk group. The pattern was repeated across all phases and for nausea control as well, he said.
“In patients receiving cisplatin-based chemotherapy, NEPA plus dexamethasone resulted in very good [chemotherapy-induced nausea and vomiting] control that was comparable between the two risk categories.… This was admittedly a post hoc analysis, but to me the bottom line is that regardless of the chemotherapy agent used in combination with cisplatin, emetic control will not deteriorate as a consequence of adding a highly emetogenic agent to cisplatin,” Dr. Hesketh concluded. ■
Disclosure: Dr. Aapro has received study grants and has been a consultant or speaker for Helsinn, Eisai, Merck, Roche, and Janssen. Dr. Schwartzberg reported no potential conflicts of interest. Dr. Jordan is on the speakers bureau and advisory board of Helsinn and Merck. Dr. Hesketh is a noncompensated consultant for Helsinn.
References
1. Aapro M, Rugo H, Rossi G, et al: A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy. Ann Oncol 25:1328-1333, 2014.
2. Hesketh PJ, Rossi G, Rizzi G, et al: Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy: A randomized dose-ranging pivotal study. Ann Oncol 25:1340-1346, 2014.
3. Gralla R, Bosnjak S, Hontsa A, et al: A phase 3 study evaluating the safety and efficacy of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting over repeated cycles of chemotherapy. Ann Oncol 251:1328-1333, 2014.
4. Aapro M, Karthaus M, Schwartzberg L, et al: Multiple, cycle CINV control and safety of NEPA, a capsule containing netupitant and palonosetron administered once per cycle of moderately emetogenic chemotherapy. MASCC/ISOO International Symposium on Supportive Care in Cancer. Abstract 64. Presented June 26, 2014.
5. Schwartzberg L, Aapro M, Hesketh PJ, et al: Do NK1 receptor antagonists contribute to nausea control? Evaluation of the novel NEPA fixed-dose combination of NK1 RA + 5-HT3 RA from pivotal trials. MASCC/ISOO International Symposium on Supportive Care in Cancer. Abstract 81. Presented June 26, 2014.
6. Jordan K, Gralla R, Rossi G, et al: Is the addition of an NK1 receptor antagonist beneficial in patients receiving carboplatin? Supplementary data with NEPA, a fixed-dose combination of netupitant and palonosetron. MASCC/ISOO International Symposium on Supportive Care in Cancer. Abstract 82.
7. Hesketh PJ, Gralla RJ, Rossi G, et al: NEPA, a fixed-dose antiemetic combination of netupitant and palonosetron: Results of effectiveness in 407 patients receiving cisplatin plus chemotherapy of various emetic risk. MASCC/ISOO International Symposium on Supportive Care in Cancer. Abstract 80. Presented June 26, 2014.