
Optimizing immune function is the new therapeutic frontier in Hodgkin lymphoma. Aside from PD-1 [programmed cell death protein 1], there are other immune targets. Many immune therapies will be going front line.— Stephen M. Ansell, MD, PhD
Tweet this quote
In the treatment of classical Hodgkin lymphoma, antibodies targeting programmed cell death protein 1 (PD-1) are just the beginning, according to Stephen M. Ansell, MD, PhD, Professor of Medicine and Chair of the Lymphoma Group at the Mayo Clinic, Rochester, Minnesota.1 Speaking at the 2016 Pan Pacific Lymphoma Conference, Dr. Ansell commented, “We are at a very exciting time. Optimizing immune function is the new therapeutic frontier in Hodgkin lymphoma. Aside from PD-1, there are other immune targets. Many immune therapies will be going front line.”
Why Immunotherapy?
Describing the pathophysiology of Hodgkin lymphoma, Dr. Ansell noted that just 1% to 2% of cells are Reed-Sternberg cells (ie, neoplastic cells that consistently express CD30 antigens). Along with “driving the bus,” Reed-Sternberg cells also secrete molecules that recruit a plethora of immune cells into the microenvironment.
“Targeting CD30 is important, but that’s just targeting a minority of cells,” explained Dr. Ansell. In developing precision immunologic agents, it will be necessary to understand the interactions between the tumor and the microenvironment. Ultimately, the microenvironment can also be targeted.
Epstein-Barr virus plays an interesting role in Hodgkin lymphoma; for one thing, Epstein-Barr virus and CIITA translocations increase the expression of programmed cell death ligands 1 and 2 (PD-L1/2). Alterations in chromosome 9p24.1 also increase the expression of PD-L1 and PD-L2.
Suppressing PD-L1 and PD-L2 is a therapeutic opportunity that is already being exploited. “But we have lots to understand about what’s going on here,” commented Dr. Ansell. “The question is, when we block PD-L1/2 signaling, who is doing the business of taking care of the cancer cell? It’s not been clarified yet.”
Immune checkpoint therapy works “very well” in classical Hodgkin lymphoma by blocking PD-1 or PD-L1/2 and essentially “waking up” the suppressed effector cells. This process makes them more active and more able to target the tumor cell.
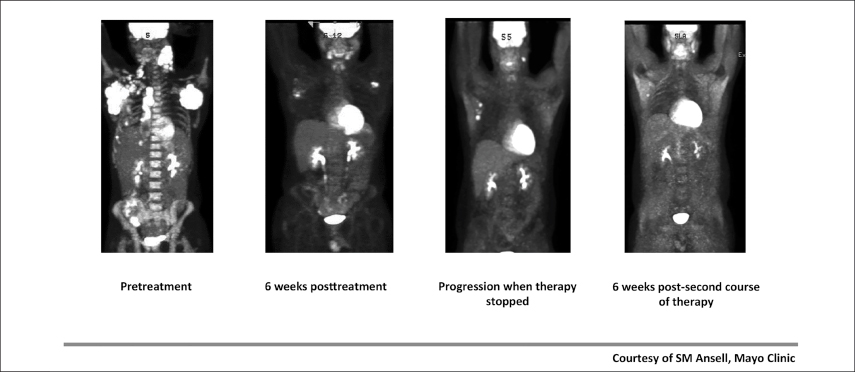
Fig. 1: Initial Therapy and Retreatment With Anti–PD-1 Therapy.
Dr. Ansell has treated a number of patients with active disease and has seen fairly dramatic responses (Fig. 1), including responses in patients with “massive adenopathy” and those “headed for hospice.”
Nivolumab Population
In a phase I study of nivolumab (Opdivo) in 23 patients, virtually every patient benefited, with a “very encouraging” overall response rate of 87%, noted Dr. Ansell.2 At a median follow-up of 86 weeks, many of these responses were sustained, and most patients were continuing to benefit.3
Median progression-free survival has not yet been reached in this study. Overall survival is 91% at 1 year and 83% at 1.5 years, with “the majority of patients remaining alive,” he added.
At the 2015 Annual Meeting of the American Society of Hematology (ASH), Dr. Ansell reported that of 20 responders, 10 have had durable responses, some lasting more than 96 weeks at the time of the analysis.3 Responses were maintained in two patients after discontinuing nivolumab. Eight patients with durable responses have received nivolumab for more than 1 year, including 7 patients responding for more than 1.5 years. Several patients achieved remission and underwent stem cell transplant, he reported.
Overall, 3 patients discontinued nivolumab due to adverse events, including grade 2 peripheral neuropathy, grade 3 myelodysplastic syndrome, and grade 3 pancreatitis. Grade 1 or 2 immune-related adverse events occurred in 4 of these 10 long-term responders and resolved without treatment in 2 patients.
“We wondered if the toxicity might become unmanageable, and it was gratifying to learn that blocking PD-1 over time does not result in profound immune complications,” Dr. Ansell said. “With nivolumab, fatigue is one of the longer-term side effects, and the others pertain to the body interacting with the environment,” he added.
Pneumonitis, skin rash, bowel issues, and autoimmune-related phenomena are the primary complaints. “These seem stable over time. They don’t necessarily turn into something that is unmanageable as treatment continues,” Dr. Ansell indicated.
A key question has been whether patients who stop treatment can benefit from retreatment upon disease progression. It seems likely, reported Dr. Ansell, describing a 26-year-old partial responder who remained on nivolumab for 2 years before coming off the drug, as mandated by the trial protocol. He developed new lesions at 8 months, was restarted on the drug, and achieved a subsequent response.
“This is good news and bad news. We can re-treat and get a very effective second response,” revealed Dr. Ansell. “But the bad news is there is no immunologic memory. You would have thought that if the T cells had the tumor cell’s ‘number,’ the next time the cancer showed up, they would take out the tumor cells, but they did not.”
Pembrolizumab Population
“Equally encouraging are the pembrolizumab [Keytruda] data,” Dr. Ansell said. In 29 heavily pretreated patients, the response rate was 66%, and the median duration of response was not reached, in a study by Moskowitz et al.4 The clinical benefit rate (including stable disease) was 86%, and most responses were durable for about 2 years.3
“The vast majority of patients had excellent responses,” reported Dr. Ansell. “The 66% response rate may be more reflective of what’s really happening with this kind of treatment.”
Grade 3 or higher treatment-related adverse events were rare and included axillary pain, hypoxia, joint swelling, and pneumonitis in one patient each. There were no grade 4 events or treatment-related deaths.
The fact that PD-L1 was highly expressed in the patients, vs controls, confirms that PD-L1 is a ligand highly expressed on tumor cells, and blocking it is “relevant and important,” Dr. Ansell commented.
Confirmatory Studies
At the 2016 ASCO Annual Meeting, phase II studies confirmed the activity of the anti–PD-1 agents in treatment-refractory patients. In Checkmate 205, which involved 80 patients who had received autologous stem cell transplant and brentuximab vedotin (Adcetris), nivolumab produced responses in 66%, with 6-month progression-free survival of 77% and overall survival of 99%.5 In KEYNOTE-087, pembrolizumab produced a 70% response rate after stem cell transplant and brentuximab vedotin and an 80% response rate after chemotherapy plus brentuximab vedotin.6 “Both these trials confirm that PD-1 blockade is an effective treatment for patients across the board,” declared Dr. Ansell.
Immunotherapy in Hodgkin Lymphoma
- A number of immune-targeted strategies are being developed for the treatment of classical Hodgkin lymphoma.
- First among these therapies are the anti–PD-1 agents, which are producing response rates of 66% and higher in patients with relapsed and refractory disease, with many durable responses.
- Tolerability has been acceptable with long-term treatment.
- Novel approaches include agents targeting CD47 on malignant cells and SIRPα on macrophages, which will enhance phagocytosis.
Data are limited on patients treated with an anti–PD-1 agent after allogeneic transplant, but results point to efficacy without significant worsening graft-vs-host disease. There has been one reported fatality due to this complication, “so while this is a reasonable option to consider for these patients, we should be cautious,” he advised.
Future Targets
Dr. Ansell also described the targeting of macrophages as a future treatment option in Hodgkin lymphoma. Higher expression of infiltrating CD68-positive macrophages has been associated with worse progression-free and overall survival.
“Reed-Sternberg cells will recruit monocytes and macrophages and these cells will do a lot to encourage malignant cell growth. Can we harness this?” he asked.
As a cell ages, markers develop on its surface, in essence saying “find me,” and this cell becomes vulnerable to being eliminated by phagocytic cells such as macrophages. However, the cell can remain protected from elimination by having CD47 on its surface (the “don’t-eat-me” signal). Malignant cells can “hijack” this process and thus protect themselves by increasing CD47 on their surface and delivering a similar “do not eat me” signal through CD47 binding to a SIRPα (signal regulatory protein alpha)—a critical immune inhibitory receptor on macrophages.
“CD47-SIRPα signaling modulates phagocytosis by intratumoral macrophages. The tumor thus prevents itself from being eaten,” Dr. Ansell explained.
There is the potential for therapies—either antibodies targeting CD47 or SIRPα—to block or eliminate the “do not eat me” signal. Such agents are in development, he said.
“The question is how to take these data and combine these agents with standard approaches,” concluded Dr. Ansell. “The key is still to maintain a high cure rate, decrease toxicity, and not compromise this with immunologic changes that could potentially compromise outcomes for patients.” ■
Disclosure: Dr. Ansell has received research funding from Bristol-Myers Squibb, Merck, Trillium, and Seattle Genetics.
References

