An exploratory analysis of KEYNOTE-522, which established the benefit of neoadjuvant pembrolizumab plus chemotherapy in triple-negative breast cancer, has now provided data to further describe prognosis and possibly guide treatment.1 In the study, presented at the 2022 ASCO Annual Meeting, event-free survival was worse for patients with higher residual cancer burden (RCB) scores. However, treatment with pembrolizumab plus chemotherapy has “shifted” the RCB toward less residual disease, according to Lajos Pusztai, MD, DPhil, Professor of Medicine and Director of Breast Cancer Research at Yale School of Medicine.

“The addition of pembrolizumab not only increased the pathologic complete response rate but shifted residual cancer burden to lower categories across the entire spectrum.”— Lajos Pusztai, MD, DPhil
Tweet this quote
“There were a lower percentage of patients in each RCB category in the pembrolizumab group than the placebo group. This indicates that the addition of pembrolizumab not only increased the pathologic complete response rate but shifted RCB to lower categories across the entire spectrum of residual disease,” he reported. “Also, the addition of pembrolizumab resulted in numerically fewer event-free survival events in the RCB-0 (complete pathologic response), RCB-1 (measurable residual disease), and RCB-2 (moderate amount of residual cancer) categories, with the most pronounced benefit seen in the RCB-2 group.”
Treatment with pembrolizumab plus chemotherapy led to 3-year event-free survival rates of almost 95% for those with an RCB score of 0 and 76% and 85% for those with an RCB score of 1 and 2, respectively. The smallest subset with extensive residual disease (RCB 3) in both arms (5.1% and 6.7%, respectively) had a poor outcome; the 3-year event-free survival rates were 26% and 35%, respectively.
“These results indicate that the event-free survival benefit from pembrolizumab extends to patients who do not achieve a pathologic complete response and suggest there is a contribution from the adjuvant pembrolizumab component of the treatment,” Dr. Pusztai suggested.
KEYNOTE-522 Background
KEYNOTE-522 tested the benefit of adding pembrolizumab to chemotherapy in 1,174 patients with early-stage triple-negative breast cancer. After definitive surgery, patients also received pembrolizumab or placebo for nine cycles or until recurrence or unacceptable toxicity.
The primary results showed statistically significant and clinically meaningful improvements in the pathologic complete response rate (a 13.6% absolute increase; P = .00055)2 and event-free survival (hazard ratio [HR] = 0.63; P = .00031) with pembrolizumab.3 The findings led to approval in the United States and Europe for pembrolizumab plus chemotherapy as neoadjuvant therapy, followed by adjuvant single-agent pembrolizumab, in high-risk patients with early-stage triple-negative disease.
Prior studies have shown the prognostic value of RCB score and RCB categories in quantifying the extent of residual disease after neoadjuvant chemotherapy.4 The current exploratory analysis of KEYNOTE-522 assessed event-free survival according to RCB category. The median follow-up was 39.1 months.
Downward Shift in RCB Category With Pembrolizumab
RCB was assessed by a local pathologist at the time of surgery, and it was categorized as RCB-0 (equivalent to a pathologic complete response), RCB-1, RCB-2, and RCB-3, reflecting increasingly larger residual cancer. The researchers evaluated event-free survival by treatment arm within RCB categories in all patients in KEYNOTE-522; hazard ratios were calculated using a Cox regression model with treatment as a covariate.
Reporting the prevalence of RCB categories by treatment arm in all patients, Dr. Pusztai commented: “In addition to the increase in the proportion of patients in the RCB-0 (or pathologic complete response) category in the pembrolizumab arm (63.4% vs 56.2%), there were also fewer patients in the RCB-1, -2, and -3 categories in the pembrolizumab arm. These findings indicate that the addition of pembrolizumab shifted the RCB categories to lower values across the entire spectrum of residual disease.”
KEY POINTS
- An exploratory analysis of KEYNOTE-522 examined residual cancer burden (RCB) scores in patients with early-stage triple-negative breast cancer treated preoperatively with pembrolizumab plus chemotherapy.
- RCB was “shifted” downward with pembrolizumab/chemotherapy, meaning fewer patients had extensive residual disease.
- RCB scores were found to be associated with event-free survival.
- In all but the highest RCB category, pembrolizumab/chemotherapy resulted in better event-free survival.
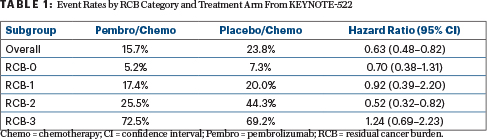
Of note, higher RCB scores were associated with worse event-free survival. The recurrence event rates and hazard ratios with 95% confidence intervals by RCB category and treatment arm are shown in Table 1.
The most common event in both arms was distant recurrence, which occurred in fewer patients receiving pembrolizumab in all RCB categories. Distant recurrence rates were 3.2% vs 5.5% in the RCB-0 group, 8.7% vs 8.9% in the RCB-1 group, 15.2% vs 22.8% in RCB-2, and 35% vs 53.8% in the RCB-3 group.
“These results highlight the importance of neoadjuvant treatment with pembrolizumab for improving survival in patients with early-stage triple-negative breast cancer and also identified a subset for whom additional therapies will be needed, those with moderate and extensive residual disease.”
Audience Question
During the discussion period, it was pointed out that the RCB-0 and RCB-1 groups had very good event-free survival, even with chemotherapy alone. The question was raised whether this group needs adjuvant pembrolizumab.
“These subgroups have too few patients to be able to draw a statistically sound conclusion about differences between the trial arms in these subsets. As illustrated in Table 1, the rate of recurrence was numerically lower in all RCB groups with pembrolizumab, except in the small RCB-3 subset,” Dr. Pusztai said. “I would also remind you that the immunologic side effects are front-loaded. Most of the immunologic adverse events happened in the neoadjuvant phase of the study; in the adjuvant phase, pembrolizumab was relatively well tolerated…. Not adding adjuvant pembrolizumab is risky, since the recurrence rate is still 15% in the RCB-1 group…, but currently planned future studies will be addressing the value of adjuvant pembrolizumab in patients with a pathologic complete response/RCB-0.”
DISCLOSURE: Dr. Pusztai has reported financial relationships with Athenex, BioTheranostics, Natera, OncoCyte, Almac Diagnostics, AstraZeneca, Bristol Myers Squibb, Clovis Oncology, Eisai, H3 Biomedicine, Immunomedics, Merck, Novartis, Pfizer, Roche/Genentech, Seattle Genetics, and Syndax.
REFERENCES
1. Pusztai L, Denkert C, O’Shaughnessy J, et al: Event-free survival by residual cancer burden after neoadjuvant pembrolizumab + chemotherapy vs placebo + chemotherapy for early-stage TNBC: Exploratory analysis from KEYNOTE-522. 2022 ASCO Annual Meeting. Abstract 503. Presented June 7, 2022.
2. Schmid P, Cortes J, Pusztai L, et al: Pembrolizumab for early triple-negative breast cancer. N Engl J Med 382:810-821, 2020.
3. Schmid P, Cortes J, Dent R, et al: Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med 386:556-567, 2022.
4. Yau C, Osdoit M, van der Noordaa M, et al: Residual cancer burden after neoadjuvant chemotherapy and long-term survival outcomes in breast cancer: A multicentre pooled analysis of 5161 patients. Lancet Oncol 23:149-160, 2022.

