
“This is the first clinical trial to have the statistical power to show that ribociclib has clinical benefit specifically in this population [premenopausal patients with hormone receptor–positive, HER2-negative breast cancer].”— Debu Tripathy, MD
Tweet this quote
PREMENOPAUSAL WOMEN with hormone receptor–positive, HER2-negative advanced breast cancer benefited substantially from the addition of ribociclib (Kisqali) to first-line endocrine therapy plus medical ovarian suppression, according to results from the MONALEESA-7 study.1 At the 2017 San Antonio Breast Cancer Symposium, Debu Tripathy, MD, reported the addition of ribociclib to standard endocrine therapy in these younger women led to a doubling in median progression-free survival. Dr. Tripathy is Chair of the Department of Breast Medical Oncology at The University of Texas MD Anderson Cancer Center, Houston.
MONALEESA-7 represents the first phase III trial dedicated to the evaluation of a cyclin-dependent kinase (CDK) 4/6 inhibitor-based regimen as front-line treatment for premenopausal women with hormone receptor–positive, HER2-negative advanced breast cancer—a population Dr. Tripathy considers “understudied.”
“There is a clear unmet need in premenopausal patients with hormone receptor–positive, HER2-negative breast cancer. It’s estimated that in this country about 20% of breast cancers are diagnosed in women under the age of 50, and these younger patients have a different biology, with more aggressive features. In fact, the last randomized trial testing a treatment in premenopausal patients was done almost 2 decades ago,” he noted.
“This is the first clinical trial to have the statistical power to show that ribociclib has clinical benefit specifically in this population. It is also the first trial to show that ribociclib can be safely and effectively combined with either tamoxifen or a nonsteroidal aromatase inhibitor together with ovarian suppression using goserelin,” Dr. Tripathy said.
In MONALEESA-7, which evaluated 672 patients, women who received ribociclib had a median progression-free survival of 23.8 months, vs 13.0 months with endocrine therapy and ovarian suppression alone, by investigator assessment (hazard ratio [HR] = 0.553; P = .0000000983). Patients in the ribociclib arm also experienced a clinically meaningful improvement in the time to deterioration of quality of life and improvement in pain scores, Dr. Tripathy reported.
Ribociclib is one of three approved endocrine therapies in postmenopausal women with advanced breast cancer. This is the first evaluation of this class of drugs in a trial that enrolled only premenopausal women. In the previous PALOMA-3 trial, 30% of subjects were premenopausal, and they derived benefit from palbociclib (Ibrance) plus fulvestrant with medical ovarian suppression, but a dedicated trial has been needed, he said.
MONALEESA-7 Details
IN MONALEESA-7, 672 premenopausal women (median age, 44 years) with advanced hormone receptor–positive, HER2-negative breast cancer were treated with either a nonsteroidal aromatase inhibitor (letrozole) or tamoxifen plus goserelin (Zoladex) and were randomized to receive ribociclib or placebo. Approximately three-quarters received an aromatase inhibitor as their endocrine therapy.
The median progression-free survival benefit shown in the primary analysis (HR = 0.553) was echoed by the blinded independent review, which found median progression-free survival that was not reached in the ribociclib arm, compared with 11.1 months in the control arm (HR = 0.427).
“Treatment benefit with ribociclib was consistent across patient subgroups and regardless of the endocrine partner [Table 1],” Dr. Tripathy reported.
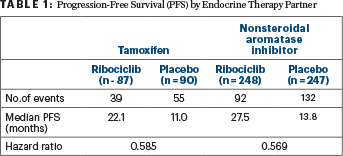
TABLE 1 : Progression-Free Survival (PFS) by Endocrine Therapy Partner
Objective response rates, for all patients, were 40.9% with ribociclib vs 29.7% with placebo (P = .00098), and for patients with measurable disease, they were 50.9% and 36.4%, respectively (P = .000317).
In addition, there was a sustained improvement in the time to definitive deterioration of at least 10% for the European Organisation for Research and Treatment of Cancer global health status/ quality-of-life scale (QLQ-C30) in the ribociclib arm, vs placebo (HR = 0.699; P = .004). A clinically meaningful (> 5 points) improvement from baseline in pain scores was observed as early as 8 weeks in patients receiving ribociclib, and it was sustained, he said.
Safety Profile
AS EXPECTED, the most frequent adverse event was neutropenia, which occurred in 76% of the ribociclib arm compared with 8% of the placebo arm, and it was grade 3/4 in 61% and 4%, respectively. In most patients, neutropenia was asymptomatic and was associated with fever in 2% and 1%, respectively, he said.
Prolongation of the QT interval (QTcF > 480 msec) occurred in 7% of the ribociclib arm vs 1% of the placebo arm, and 0.3% and 0.6% of patients, respectively, discontinued treatment as a result. No clinical consequences or arrhythmias were associated with these events. Dr. Tripathy acknowledged that prolongation of the QT interval is being observed more often with the newer small molecules as well as many other drugs that affect cardiac repolarization.
Other adverse events included hot flashes, nausea, leukopenia, and joint pain or stiffness. Adverse events leading to permanent discontinuation of treatment occurred in 4% of the ribociclib arm and 3% of the control arm.
‘A Momentous Study’

Steven Vogl, MD
STEVEN VOGL, MD, of the Bronx, New York, called MONALEESA-7 “a momentous study that should immediately change the standard of care for symptomatic young women with metastatic breast cancer.” He noted that with the regimen of endocrine therapy, goserelin, and ribociclib, “more and more of the patients get better and stay better longer…These are people for whom we are trying to buy some good life, before the inevitable happens.”
Dr. Vogl commented that the study is also noteworthy for being the first to evaluate the use of ovarian suppression incorporating an aromatase inhibitor in premenopausal patients. “You showed that the addition of the CDK4/6 inhibitor is both effective and tolerable, and that in a patient who is tamoxifen-naïve, you can give tamoxifen or an aromatase inhibitor with ovarian suppression and the CDK4/6 inhibitor,” he said.
Dr. Tripathy acknowledged the two options provide “flexibility,” which is important when considering toxicities experienced with one or the other endocrine agents. “We were glad to see equivalent results between the aromatase inhibitor and tamoxifen. Even at the first observation time, 8 weeks, we saw a clear separation of the curves [with ribociclib vs placebo],” he said.
“Most of us would start with the aromatase inhibitor first, but I believe these drugs can sometimes cause more symptoms than tamoxifen. It’s good to have flexibility,” he said.
Additional Commentary

Howard A. “Skip” Burris III, MD
HOWARD A “SKIP” BURRIS III, MD, Chief Medical Officer and President of Clinical Operations at Sarah Cannon Research Institute, Nashville, echoed Dr. Tripathy’s sentiments regarding the need to evaluate treatments in the premenopausal population: “We have been dodging the premenopausal patient question. We think of estrogen receptor–positive disease as being in 65-year-olds, but in women under 50, it can be more aggressive. New therapies have been needed for these women, who have been neglected in clinical trials.”
In Dr. Burris’ view, the MONALEESA-7 data “look very powerful,” showing ribociclib may work even better in the more challenging premenopausal patients. He also applauded the investigators for conducting this study, since the discussion of ovarian suppression regimens and so forth can be time-consuming in terms of patient visits.
Dr. Burris also said the results confirm the “hassle of ovarian suppression” can, indeed, be “worth the gain” when a drug like ribociclib is added. “The impact isn’t a little bit; it’s a lot.” ■
DISCLOSURE: Drs. Tripathy, Vogl, and Burris reported no conflicts of interest.
REFERENCE
1. Tripathy D, Sohn J, Im S-A, et al: First-line ribociclib vs placebo with goserelin and tamoxifen or a non-steroidal aromatase inhibitor in premenopausal women with hormone receptor-positive, HER2-negative advanced breast cancer: Results from the randomized phase III MONALEESA-7 trial. 2017 San Antonio Breast Cancer Symposium. Abstract GS2-05. Presented December 6, 2017.

