THE PHASE III SUCCESS A trial, presented at the 2017 San Antonio Breast Cancer Symposium, found no benefit for extending the use of intravenous zoledronic acid from 2 years to 5 years.1
“At this time point, our study showed no difference in disease-free survival or overall survival between 5 years and 2 years of adjuvant [zoledronic acid] treatment following adjuvant chemotherapy in patients with high-risk early breast cancer, irrespective of menopausal status,” said Wolfgang Janni, MD, PhD, of University Hospital Ulm in Germany. “Five years of treatment should not be considered currently in these patients, in the absence of decreased bone density.

“Our study showed no difference in disease-free survival or overall survival between 5 years and 2 years of adjuvant [zoledronic acid] treatment following adjuvant chemotherapy in patients with high-risk early breast cancer, irrespective of menopausal status.”— Wolfgang Janni, MD, PhD
Tweet this quote
Bisphosphonates have been shown to reduce skeletal events in cancer patients, prevent recurrences to the bone, and improve survival, especially in postmenopausal patients. The optimal duration of treatment has been unclear, Dr. Janni said. In 2017, ASCO and Cancer Care Ontario recommended zoledronic acid (or clodronate) every 6 months for 3 to 5 years in postmenopausal patients with breast cancer.2
Julie Gralow, MD, Director of Breast Medical Oncology for the Seattle Cancer Care Alliance and an expert on bone disease in breast cancer, said the findings of the SUCCESS A trial are “not definitive” and advised tailoring treatment. “What the study says is that maybe not every patient needs a full 5 years of adjuvant bisphosphonates, but I don’t think we have answered the question about optimal duration.”
SUCCESS A Details
THE OPEN-LABEL multicenter phase III study recruited 3,754 women with high-risk early breast cancer (> 70% hormone receptor–positive), randomizing them first to adjuvant chemotherapy (3 cycles of FEC [fluorouracil, epirubicin, cyclophosphamide]) followed by 3 cycles of docetaxel alone or docetaxel with gemcitabine. After chemotherapy, they were re-randomized to either 2 or 5 years of zoledronic acid at 4.0 mg intravenously every 3 months for the first 2 years in each arm, and 4.0 mg every 6 months for the remaining 3 years in the 5-year treatment arm. The study was completed by 1,540 patients in the 5-year arm and 1,447 in the 2-year arm.
At a median follow-up of about 3 years, investigators found no significant differences in outcomes:
- Disease-free survival was the same in each arm—approximately 90% at 40 months; there were 129 events in the 5-year arm and 121 events in the 2-year arm (P = .827).
- Disease-free survival rates did not differ according to menopausal status.
- Overall survival was the same per arm—approximately 95%—based on 59 events in the 5-year arm and 57 in the 2-year arm (P = .713).
- Bone recurrences (as the first distant recurrence) also did not differ, occurring in 25 patients in the 5-year arm and 28 in the 2-year arm.
- The multivariable analysis adjusted for age, body mass index, menopausal status, tumor size, nodal stage, histologic grade, histologic type, hormone receptor status, HER2 status, type of surgery, and adjuvant chemotherapy. The hazard ratios for 5 vs 2 years were 0.97 (P = .81) for disease-free survival and 0.98 (P = .90) for overall survival.
Adverse Events More Frequent With 5 Years
FIVE YEARS of the drug, however, was associated with an increased frequency of adverse events, vs 2 years. Table 1 shows adverse events as of 2 years after the start of zoledronic acid.
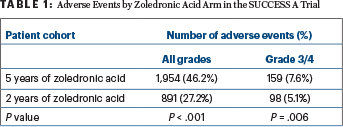
The greatest differences were seen with bone pain (all grades, 8.3% of the 5-year arm vs 3.7% of the 2-year arm), arthralgia (5.1% vs 3.1%), fatigue (4.4% vs 2.1%), anemia (4.4% vs 0.5%), leukopenia (3.6% vs 0.6%), and elevation of serum glutamic pyruvic transaminase (2.5% vs 0.7%). Grade 3/4 adverse events, however, were rare in both arms.
Circulating Tumor Cells
CIRCULATING TUMOR cells have prognostic relevance, and according to one hypothesis, bisphosphonates could play a role in eliminating them. In 714 patients, investigators took blood samples after chemotherapy and after 2 and 5 years of zoledronic acid to assess changes in their density. In the 5-year arm, 10.5% of patients were circulating tumor cell–positive, compared to 7.2% of the 2-year arm (P = .135).
“We saw a lack of significant difference with regard to the prevalence of [circulating tumor cells] 5 years after adjuvant chemotherapy, in accordance with the survival analysis,” Dr. Janni said.
Dr. Janni acknowledged some limitations of the SUCCESS A trial. Median observation time is limited, the 2-year arm had more patients lost to follow-up (though median observation time for these patients was not significantly different from the 5-year arm); and the number of events was low.
DISCLOSURE: Dr. Janni has received a research grant from Novartis. Dr. Gralow has served as a consultant for Genentech/Roche, Novartis, Merck, Pfizer, Sandoz, Puma, and AstraZeneca.
REFERENCES
1. Janni W, et al: Extended adjuvant bisphosphonate treatment over five years in early breast cancer does not improve disease-free and overall survival compared to two years of treatment. 2017 San Antonio Breast Cancer Symposium. Abstract GS1-06. Presented December 6, 2017.
2. Dhesy-Thind S, et al: Use of adjuvant bisphosphonates and other bone-modifying agents in breast cancer: A Cancer Care Ontario and American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 35:2062-2081, 2017.


