The World Health Organization system organizes myeloid malignancies into five major categories, which are subsequently further subclassified using a combination of bone marrow morphology and cytogenetic/molecular information (Table 1).1 JAK2 and MPL mutations are not disease-specific and occur across the spectrum of myeloid malignancies, including BCR/ABL1-negative myeloproliferative neoplasms (MPN), myelodysplastic syndromes (MDS), MDS/MPN overlap, and acute myeloid leukemia. These mutations are usually, but not always, absent in chronic myeloid leukemia.
The World Health Organization system organizes myeloid malignancies into five major categories, which are subsequently further subclassified using a combination of bone marrow morphology and cytogenetic/molecular information (Table 1).1 JAK2 and MPL mutations are not disease-specific and occur across the spectrum of myeloid malignancies, including BCR/ABL1-negative myeloproliferative neoplasms (MPN), myelodysplastic syndromes (MDS), MDS/MPN overlap, and acute myeloid leukemia. These mutations are usually, but not always, absent in chronic myeloid leukemia.
JAK2 Mutations
JAK2V617F is the most frequent mutation in BCR/ABL1-negative MPN and is present in > 95% of patients with polycythemia vera or post–polycythemia vera myelofibrosis and 60% of those with essential thrombocythemia, post–essential thrombocythemia myelofibrosis, or primary myelofibrosis. JAK2V617F is also found in approximately 50% of patients with a specific MDS/MPN entity referred to as “refractory anemia with ring sideroblasts and thrombocytosis (RARS-T),” but mutational frequency is less than 5% in acute myeloid leukemia, MDS, or other myeloid malignancies including chronic myelomonocytic leukemia.
 JAK2 mutations other than JAK2V617F (eg, JAK2 exon 12 mutations) occur in approximately 50% of patients with JAK2V617F-negative polycythemia vera. In almost all instances, polycythemia vera cases with JAK2 exon 12 mutations display subnormal serum erythropoietin levels. MPL mutational frequencies are estimated at 8% in primary myelofibrosis and 4% in essential thrombocythemia, but they also infrequently occur in acute megakaryoblastic leukemia, RARS-T, and MDS with del(5q). Neither JAK2 nor MPL mutations are found in healthy people or those with secondary polycythemia, reactive thrombocytosis, lymphoid malignancies, or solid tumors.
JAK2 mutations other than JAK2V617F (eg, JAK2 exon 12 mutations) occur in approximately 50% of patients with JAK2V617F-negative polycythemia vera. In almost all instances, polycythemia vera cases with JAK2 exon 12 mutations display subnormal serum erythropoietin levels. MPL mutational frequencies are estimated at 8% in primary myelofibrosis and 4% in essential thrombocythemia, but they also infrequently occur in acute megakaryoblastic leukemia, RARS-T, and MDS with del(5q). Neither JAK2 nor MPL mutations are found in healthy people or those with secondary polycythemia, reactive thrombocytosis, lymphoid malignancies, or solid tumors.
Indications for Screening
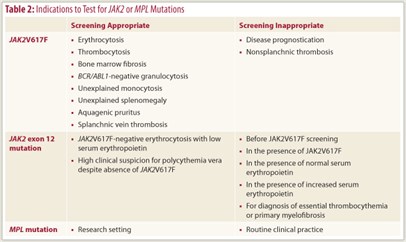
Table 2 on page 46 outlines the indications for JAK2 or MPL mutation screening in routine clinical practice. Because JAK2V617F is present in the majority of patients with polycythemia vera, essential thrombocythemia, or primary myelofibrosis, it is reasonable to consider its screening during the evaluation of erythrocytosis, thrombocytosis, bone marrow fibrosis, or other symptoms that are characteristic of these diseases (eg, splanchnic vein thrombosis, aquagenic pruritus, and otherwise unexplained splenomegaly or leukocytosis). It is important to note that one does not require an abnormal blood count to consider JAK2V617F mutation screening in patients with splanchnic vein thrombosis. Results are the same regardless of whether JAK2V617F screening was performed using peripheral blood or bone marrow.
JAK2 exon 12 and other exon mutations occur mostly in patients with JAK2V617F-negative polycythemia vera, and they are almost always associated with subnormal serum erythropoietin level. Therefore, their screening is warranted only when one suspects JAK2V617F-negative polycythemia vera and the serum erythropoietin level is subnormal. In other words, screening for JAK2 exon 12 mutations before screening for JAK2V617F or in the presence of normal or increased serum erythropoietin level is discouraged.
The incidences of MPL mutations in MPN are too low to warrant their use in routine clinical practice. The reason I recommend JAK2 exon 12 mutation screening in special circumstances (see above) whereas I do not recommend MPL mutation screening in routine clinical practice is that the two techniques have a different impact on management: Detecting mutant MPL has no additional diagnostic value since morphology is adequate in diagnosing primary myelofibrosis or essential thrombocythemia, whereas the diagnosis of JAK2V617F-negative polycythemia vera is therapeutically important.
I prefer using cell-based quantitative assays for JAK2V617F mutation screening for a number of reasons: (1) diagnostic certainty is enhanced in the presence of > 1% mutant allele burden and anything less than < 0.1% mutant allele burden should be considered negative until proven otherwise, (2) a ≥ 50% JAK2V617F allele burden suggests polycythemia vera or prefibrotic myelofibrosis as opposed to essential thrombocythemia, and (3) quantitative measurements are more suitable for monitoring response to treatment known to affect mutant allele burden (eg, interferon-α) or assessing minimal residual disease after allogeneic stem cell transplantation. Neither the presence nor the burden of JAK2 or MPL mutation is prognostically useful, and mutation screening should not be performed for this purpose.
A positive result for either JAK2 or MPL mutation confirms the presence of a myeloproliferative neoplasm but does not distinguish one type from another. A JAK2 mutation accompanied by elevated hemoglobin is usually adequate for the diagnosis of polycythemia vera and one does not necessarily require a bone marrow examination or red cell mass measurement in addition.
The absence of a JAK2 mutation and normal or elevated serum erythropoietin level makes the diagnosis of polycythemia vera very unlikely. The absence of JAK2 or MPL mutation does not exclude either essential thrombocythemia or primary myelofibrosis. Therefore, one needs to consult with an expert hematopathologist to establish an accurate diagnosis of essential thrombocythemia or primary myelofibrosis (including prefibrotic myelofibrosis) regardless of the results of the JAK2/MPL mutation screening. ■
Disclosure: Dr. Tefferi reported no potential conflicts of interest.
Reference
1. Vardiman JW, Thiele J, Arber DA, et al: The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood 114:937-951, 2009.